
Effects of early enteral nutrition on the prognosis of patients with sepsis: secondary analysis of acute gastrointestinal injury study
Introduction
As the population ages, the incidence of sepsis is increasing, a significant healthcare problem that affects millions of people each year (1). It is defined as a dysregulated host response to the infection caused to life-threatening organ dysfunction and kills one in four (2-4). Intestinal failure is one of the crucial mechanisms of sepsis-induced multiple organ dysfunction, and it is believed the incidence of intestinal failure in septic/septic shock patients is as high as 60% (5,6). Enteral nutrition (EN) could improve clinical outcomes of critically ill patients, including decreased length of hospital stay, reduced mortality (7-9).
The direct benefits of the EN were maintained gut integrity, reduced gut permeability; other benefits were that and attenuated disease severity, modulate stress, and the systemic immune response (10-12). The optimal time to safely and deliver EN in patients receiving intravenous vasopressor support for septic shock was controversy, despite recommendations for early enteral nutrition (EEN) in most critically ill patients (13,14). The patients on vasopressors were recommended EEN may have a beneficial effect on these patients in the 2013 Canadian Critical Care practice guideline for nutrition (15). In 2016, the American Nutrition Guidelines recommended patients with severe sepsis/septic shock should be treated with enteral nutrition (EN) within 24–48 hours, a recommendation to withhold EN in patients who have hemodynamic instability (mean arterial pressure less than 50 mmHg and require initiation or escalation of vasopressors) (16). The European Society of Intensive Care Medicine (ESICM) clinical practice guidelines suggest delaying EN if the shock is uncontrolled and hemodynamic and tissue perfusion goals are not reached; low-dose EN then should be started as soon as the shock is controlled with fluids and vasopressors/inotropes. There is concern regarding the application of EN when very high doses of vasopressors (e.g., norepinephrine >1 µg/kg/min) are required, and hyperlactatemia is persistent or when other signs of end-organ hypoperfusion are present (17). As we know, it was necessary to take EEN, account for patients with sepsis on vasopressors or not—the lack of a consistent definition of EEN, including what time of EN administration was greatest. The study aimed to discuss what time of EEN could improve the prognosis of patients with sepsis. We present the following article in accordance with the STROBE reporting checklist (http://dx.doi.org/10.21037/apm-20-1650).
Methods
Study design and patients
This study was a secondary analysis of pooled data from the acute gastrointestinal injury grade study, which was a prospective, observational, multicenter study. The research was registered in the Chinese Clinical Trial Registry (ChiCTR-OCS-13003824). The primary trial endpoint and study protocol have been published previously (18). Patients were screened for eligibility within 24 hours of ICU admission. Written informed consent was retrieved from all participators before inclusion. The inclusion criteria: (I) >18 years of age; (II) acute Physiology and Chronic Health Evaluation II (APACHE II) score >8; (III) they were required to stay for at least 24 hours in the ICU; (IV) the patients with sepsis [the diagnostic criteria for sepsis, defined as sepsis-3, are infection-causing, life-threatening organ dysfunction, and Sequential Organ Failure Assessment (SOFA) ≥2]. The exclusion criteria: (I) AGI could not be tested for any reason; (II) advanced cancer; (III) any terminal stage disease. The initiation criteria for continuous EN in the ICU were guided by a multidisciplinary approach following the nutrition protocol.
The research protocol was reviewed and approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (2013KY050). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Nutrition protocol
After the patient was included in the study, according to the patient’s nutritional status and hemodynamic status, if the patient had stable hemodynamics, the patient was received EN. The EN infusion rate and the total daily depend upon the gastric residual volume (GRV) and nutrition target. The nutrition target is that 20 kcal/kg body weight/day within the first week of ICU admission. If the patient had protein-calorie malnutrition at ICU admission and EN could not reach 60% of the nutrition target, then the patient was needed to receive SPN from the fourth day of ICU admission.
Data collection
All patient data were provided in a specific case report, including baseline demographic and clinical characteristics and nutritional status, APACHE II score and Sequential Organ Failure Assessment (SOFA) score were collected within the first 24 hours of ICU admission. The primary outcome was 28- and 60-day all-cause mortality after admission. Patients who survived were followed by telephone.
Statistical methods
APACHE II, SOFA, and NRS2002 measurement data are shown as the mean ± standard deviation. The normally distributed data are determined with independent T-tests. The non-positive distribution used an analysis of variance. The count data, including survival rate and survival time, are expressed in terms of median quartile and rate, using the Mann-Whitney test and Chi-square test. The patient source used the Kruskal-Wallis test. Kaplan-Meier survival analysis was performed to estimate the 28- and 60-day cumulative survival. The log-rank test was used to compare the survival rates of different subgroups of patients. The prognostic value of the variables was assessed using a univariate and multivariate Cox proportional hazard regression model. P<0.05 was considered to show statistical significance. SOFA was initially tested as the severity of sepsis at a time point, not as a prognostic factor for mortality (19). Therefore, COX analysis did not include the SOFA in this study. The statistical software used was SPSS 22.0 and Graphpad Prism 6.0.
Results
Baseline characteristics
A total of 676 consecutive critically ill patients were recruited from 14 general ICUs. Per the inclusion and exclusion criteria, 163 patients with sepsis were included. Among the 163 patients, 79.1% were admitted for a medical reason. These patients had a median age of 70 years with a body mass index (BMI) of 20.2±5.8 kg/m2, and the APACHE II score was 20.5±7.1, SOFA score of 9.8±4.4, and NRS2002 score of 2.1±1.3. The primary demographic characteristics of patients are given in Table 1. The primary organ disorders of patients were respiratory failure (75.4%), using vasoactive drugs (49.7%), and acute kidney injury (20.2%). Among study patients, chronic obstructive pulmonary disease, coronary artery disease, and diabetes mellitus accounted for 22.7%, 16.6%, and 17.2%, respectively. Also, 137 patients (84.0%) received mechanical ventilation, and 22 (13.5%) received renal replacement therapy (RRT). Of the 163 patients, the EEN group (≤24 hours) formed 85 patients (52.1%) and the control group (>24 hours) 78 (47.9%). There were 125 patients (76.7%) receiving EN within three days and 136 patients (83.4%) receiving EN within seven days. No significant differences were observed in baseline disease severity, age, BMI, Gender, Patient Source, other Comorbidities, Complications, the number of people who CRRT or the number of people who had intravenous glucocorticoid, vasopressor, and insulin agents at baseline.
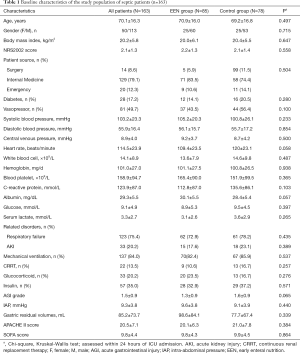
Full table
Clinical outcome
The 28- and 60-day mortality rates were 35.6% (n=58) and 45.2% (n=72), respectively. The patients in the EEN group had lower 28-day (28.2% vs. 43.6%, P=0.041) and 60-day mortality rates (36.5% vs. 52.6%, P=0.039) than those in the control group. The length of ICU stay in the EEN group was longer than in the Control group {11 [8–22] vs. 10 [6–16]; P=0.022}; Also, the length of the hospital stay was longer than in the Control group {23 [14–53] vs. 18 [10–39]; P=0.023}. The new infection rate did not differ between the two groups (Table 2).
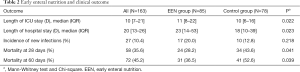
Full table
Kaplan-Meier survival analysis
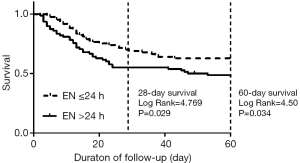
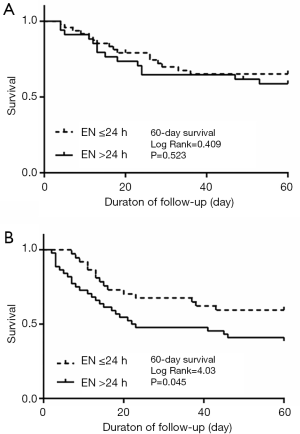
By Kaplan-Meier survival analysis, the survival curves stratified on the time of EN for 28- and 60-day mortality in the overall population (Figure 1). The survival probabilities at 28 and 60 days were 71.8% and 63.5% in the EEN group. The survival probability at 28 and 60 days were 56.4% and 47.4% in the control group. The 28-day survival probability is higher in the EEN group than in the control group (log-rank P=0.029<0.05). Comparable results were observed for 60-day mortality (log-rank P=0.034<0.05). In a subgroup analysis of patients who whether used vasoactive drugs. In the un-used population, the mortality at 60 days had no significant differences between the EEN group and the control group (P=0.523>0.05) (Figure 2A). The EEN group was found to be significantly associated with 60-day mortality (P=0.045<0.05) (Figure 2B).
Univariate and multivariate analyses for 60- day mortality and side effects
Univariate Cox regression analysis showed that EEN, using vasoactive drugs, Acute kidney injury (AKI), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and the global acute gastrointestinal injury (AGI) grade were significantly (P<0.05) associated with 60-day mortality. In a multivariate analysis including these variables, EEN (HR1.68, 95% CI: 1.02–2.62; P=0.040), global AGI grade (HR2.28, 95% CI: 1.30–4.00; P=0.004), and APACHE II score (HR 1.04, 95% CI: 1.01–1.07; P=0.021) were independently associated with 60-day mortality (Table 3). There are no significant differences in the side effects of EN between patients, whether on vasopressor, Such as diarrhea, gastrointestinal bleeding, nausea, vomiting and bloating (Table 4).

Full table

Full table
Discussion
Current studies of EEN in patients with sepsis/septic shock are still lacking, because of inconsistency in the severity of sepsis, which could cause a bias in patient selection. At present, three meta-analyses have compared early EN to the late-stage EN therapy in critically ill ICU patients (20-22). The time for early EN was 48, 36, and 24 hours. These meta-analyses found EEN can reduce mortality in critically ill patients. The American Nutrition Guidelines incorporate 21 comparisons of early and late-stage EN and standardized EN RCT studies included in re-analysis: EEN therapy can significantly reduce the mortality rate (RR =0.70; 95% CI, 0.49–1.00; P=0.05). In this study, the COX analysis of the mortality rate of early EN compared to late-stage showed that early EN, within 24 hours, can significantly reduce sepsis/septic shock in patients with 28-day mortality and 60-day mortality compared with late EN (Figure 1). EEN in patients with sepsis on vasopressors had a more significant in 60-day mortality (Figure 2B). There are no significant differences in the side effects of EN between patients, whether on vasopressor (Table 4). Because the former ICU critically ill patients included patients with sepsis/septic shock, this study found EEN outcomes in terms of mortality were consistent with those in critically ill patients. The new incidence of infection is inconsistent, probably because the original does not include intestinal dysfunction in the non-sepsis/septic shock of critically ill patients. Therefore, some basic clinical research (trauma, postoperative patients) found EEN can maintain intestinal mucosal integrity and reduce intestinal permeability and, together, can improve the intestinal immune barrier, preventing bacterial and endotoxin translocation, to reduce the incidence of secondary infection (23-25). However, intestinal dysfunction was inherent in patients with sepsis; bacterial and endotoxin translocation itself was one mechanism of sepsis. Thus, EN can treat intestinal failure and promote intestinal recovery, reducing mortality, but the infection has been present, and EN therapy, therefore, will not contribute to improving the incidence of new infections.
Simultaneously, the relationship between vascular drugs and EEN intolerance was defined as inconsistently (26-28). Mesenteric ischemia is the leading risk when administering EN to sepsis/septic shock patients who are taking vascular drugs. There is concern that EN in shock further jeopardizes the already impaired splanchnic perfusion. Mesenteric ischemia has a mortality rate of up to 80%, but an incidence of only about 0.3–3.8% (29). There was no evidence for a causal relationship between shock, vasopressors, EN, and non-occlusive mesenteric ischemia (NOMI) in some patients who suffered from NOMI (27,30,31). A recent study suggests that norepinephrine <0.14 mg/kg/min EEN was safe and tolerable in patients with sepsis/septic shock within the first 48 hours (13). Our data also shows there were no patients with mesenteric ischemia. There are no significant differences in the side effects of EN between patients, whether on vasopressor (Table 4). Therefore, EEN administration is safe when the patients with sepsis are circulatory stable. The benefits of the EEN were maintained gut integrity, reduced gut permeability.
An interesting problem was found in this study: the length of ICU stay [median time (quartile time)] of the early and late-stage EN groups, respectively, was 11 [8–22] and 10 [6–16] (P=0.022); the length of hospital stay was 23 [14–53] and 18 [10–39] (P=0.023). The length of ICU stay and length of hospital stay was longer in early EN patients than in the late-stage group, contrary to previous reports, which suggests that early EN in ICU critically ill patients reduced ICU time and length of hospital stay (22,32). Although the study did not account for the cost of hospitalization for sepsis, it is conceivable that this contradicts reports that early EEN can reduce hospital costs for ICU patients (33). We currently believe patients with sepsis in the septic shock treatment cycle should be calculated in weeks, and some even up to several months. We found that early EN therapy can significantly improve the survival rate of sepsis patients. The surviving patients certainly need time for rehabilitation, so we do not find it difficult to understand why early EN improves the survival rate of sepsis patients while leaving ICU time and the total length of stay longer in these patients than patients with late EEN. Together, we found there was no significant difference in ICU time and hospital stay between the two groups, which confirms our inference that the differences in mortality between the two groups result in the two groups having differences in ICU and hospital length of stay.
Insufficient research due to the observational nature of the study, and the sample quantity being insignificant. The initial design of this study was not considered sepsis patients. The time set in this study is time the patient enters the ICU, not the time of onset of sepsis. The relationship between shock, time, vasopressors, EN, and NOMI needs further research to explain.
Conclusions
EEN (≤24 hours) was associated with improved prognosis in patients with sepsis/septic shock. EEN was an independent prognostic factor in patients with sepsis/septic shock.
Acknowledgments
We gratefully acknowledge all the physicians, nurses, dietitians, patients, and the local investigators of each hospital involved at the 14 participating centers for their dedication to the study.
Funding: This work was supported by grants from the Public Welfare of Science Technology Department of Zhejiang Province (2014C37023), The Natural Science Foundation of Zhejiang Province (LY17H1500), The Science and Technology Project of Taizhou (1902ky02) and the Medicines Health Research Fund of Zhejiang (2019ZH062).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1650
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1650
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1650). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research protocol was reviewed and approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (2013KY050) and was registered in the Chinese Clinical Trial Registry (ChiCTR-OCS-13003824). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was retrieved from all participators.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. [Crossref] [PubMed]
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:762-74. [Crossref] [PubMed]
- Caddell KA, Martindale R, McClave SA, et al. Can the intestinal dysmotility of critical illness be differentiated from postoperative ileus? Curr Gastroenterol Rep 2011;13:358-67. [Crossref] [PubMed]
- Xu Q, Yan Q, Chen S. Use of ulinastatin was associated with reduced mortality in critically ill patients with sepsis. J Thorac Dis 2019;11:1911-8. [Crossref]
- Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg 1992;215:503-11; discussion 511-3. [Crossref] [PubMed]
- Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 1992;216:172-83. [Crossref] [PubMed]
- Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med 2005;31:12-23. [Crossref] [PubMed]
- Ammori BJ. Importance of the early increase in intestinal permeability in critically ill patients. Eur J Surg 2002;168:660-1; author reply 2. [Crossref] [PubMed]
- Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr 2007;31:246-58. [Crossref] [PubMed]
- Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998;42:431-5. [Crossref] [PubMed]
- Merchan C, Altshuler D, Aberle C, et al. Tolerability of enteral nutrition in mechanically ventilated patients with septic shock who require vasopressors. J Intensive Care Med 2017;32:540-6. [Crossref] [PubMed]
- Yang S, Wu X, Yu W, et al. Early enteral nutrition in critically ill patients with hemodynamic instability: an evidence-based review and practical advice. Nutr Clin Pract 2014;29:90-6. [Crossref] [PubMed]
- Dhaliwal R, Cahill N, Lemieux M, et al. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract 2014;29:29-43. [Crossref] [PubMed]
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.SPEN). JPEN J Parenter Enteral Nutr 2016;40:159-211. [Crossref] [PubMed]
- Reintam Blaser A, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 2017;43:380-98. [Crossref] [PubMed]
- Hu B, Sun R, Wu A, et al. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: a multicenter, prospective, observational study. Crit Care 2017;21:188. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Doig GS, Heighes PT, Simpson F, et al. Early enteral nutrition, provided within 24 h of injury or intensive care unit admission, significantly reduces mortality in critically ill patients: a meta-analysis of randomised controlled trials. Intensive Care Med 2009;35:2018-27. [Crossref] [PubMed]
- Heyland DK, Dhaliwal R, Drover JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 2003;27:355-73. [Crossref] [PubMed]
- Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 2001;29:2264-70. [Crossref] [PubMed]
- Kompan L, Vidmar G, Spindler-Vesel A, et al. Is early enteral nutrition a risk factor for gastric intolerance and pneumonia? Clin Nutr 2004;23:527-32. [Crossref] [PubMed]
- Peng L, Wu LG, Li B, et al. Early enteral nutrition improves intestinal immune barrier in a rat model of severe acute pancreatitis. J Hepatobiliary Pancreat Sci 2016;23:681-7. [Crossref] [PubMed]
- Singh G, Ram RP, Khanna SK. Early postoperative enteral feeding in patients with nontraumatic intestinal perforation and peritonitis. J Am Coll Surg 1998;187:142-6. [Crossref] [PubMed]
- Khalid I, Doshi P, DiGiovine B. Early enteral nutrition and outcomes of critically ill patients treated with vasopressors and mechanical ventilation. Am J Crit Care 2010;19:261-8. [Crossref] [PubMed]
- Mancl EE, Muzevich KM. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy. JPEN J Parenter Enteral Nutr 2013;37:641-51. [Crossref] [PubMed]
- Rai SS, O'Connor SN, Lange K, et al. Enteral nutrition for patients in septic shock: a retrospective cohort study. Crit Care Resusc 2010;12:177-81. [PubMed]
- Park WM, Gloviczki P, Cherry KJ Jr, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg 2002;35:445-52. [Crossref] [PubMed]
- Wells DL. Provision of enteral nutrition during vasopressor therapy for hemodynamic instability: an evidence-based review. Nutr Clin Pract 2012;27:521-6. [Crossref] [PubMed]
- Flordelís Lasierra JL, Perez-Vela JL, Umezawa Makikado LD, et al. Early enteral nutrition in patients with hemodynamic failure following cardiac surgery. JPEN J Parenter Enteral Nutr 2015;39:154-62. [Crossref] [PubMed]
- Nguyen NQ, Fraser RJ, Bryant LK, et al. The impact of delaying enteral feeding on gastric emptying, plasma cholecystokinin, and peptide YY concentrations in critically ill patients. Crit Care Med 2008;36:1469-74. [Crossref] [PubMed]
- Doig GS, Simpson F. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a full economic analysis of a multicenter randomized controlled trial based on US costs. Clinicoecon Outcomes Res 2013;5:369-79. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


