
Electromagnetic navigation-assisted percutaneous endoscopic foraminoplasty and discectomy for lumbar disc herniation: technical note and preliminary results
Introduction
Lumbar disc herniation (LDH) is a common degenerative disc disease, with an incidence between 3.7% and 5.1% (1). For LDH patients who have failure of conservative treatment, surgery should be performed to relieve nerve compression. Percutaneous endoscopic lumbar discectomy (PELD) is gradually becoming a new gold standard surgical procedure for LDH. Its advantages include desirable satisfactory clinical outcome, less trauma, less bleeding, less postoperative pain, and faster recovery (2-5). However, the steep learning curve is still a major technical obstacle of PELD. Multiple intraoperative fluoroscopy is needed to identify the position of the working tube for safety, and excessive X-ray exposure may expose doctors and patients to potential radiation (6).
The electromagnetic navigation-guided technique is an innovative procedure that provides navigational assistance coupled with steer ability and movability. It has been applied in neurosurgery, otolaryngology, as well as oral and maxillofacial surgery (7-9). Electromagnetic navigation has recently been applied in spinal surgery for pedicle screw placement (10,11). However, to the best of our knowledge, the application of electromagnetic navigation to guide spinal endoscopic surgery has not been reported. Therefore, we introduce a new technique of electromagnetic navigation-assisted percutaneous endoscopic lumbar foraminoplasty and discectomy using the I-See device (SEESSYS) for full visualization of the entire surgical procedure. In the present study, we describe the SEESSYS procedure, evaluate the clinical efficacy of continuous 17 cases, and outline the challenges of the widespread implementation of this new technology. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1956).
Methods
Patient selection
Consecutive patients with clinically symptomatic LDH undergoing SEESSYS procedure from September to November 2018 were included in the study. The indication for surgery was as follows: (I) typical lower-extremity radiating pain, with or without lower back pain; (II) positive straight-leg raise test, with/without muscle strength decrease or hypoesthesia of nerve root innervation area; (III) magnetic resonance imaging and computerized tomography (CT) scan indicating LDH; (IV) imaging manifestations were consistent with clinical symptoms in terms of the side and level of the herniated disc; (V) age >18 years; and (VI) provided informed consent to participate in the study. The exclusion criteria were as follows: (I) lumbar hyperextension and hyperflexion X-ray indicating segmental instability of the same diseased segment; (II) multiple LDHs; (III) previous surgical history of the same diseased segment; (IV) concomitant tumor, tuberculosis, infection, or fracture and other disease; and (V) patients with psychiatric disorders or those who could not complete the scale. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committees of Guangdong Provincial Hospital of Chinese Medicine (approval No. ZE2019-285-01). Written informed consent was obtained from participants.
Surgical tools
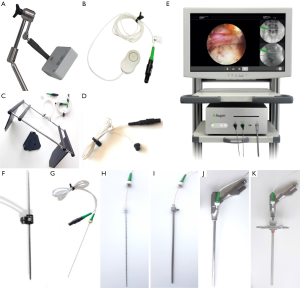
The electromagnetic navigation system (Fiagon GmbH, Germany) included the magnetic field generator, MultiPad, MaperBrige, localizer, computer mainframe and monitor, Kirschner wire, and IseePointer (Figure 1A,B,C,D,E,F,G). The I-See endoscopic spine surgical system (Joimax, IseeU, Germany) included the needle, guide rod, endoscopy, and Isee-reamer with IseePointer (Figure 1H,I,J,K). The multifunctional plasma radiofrequency electrode system (Xi’an Surgical Medical Technology, China) was also used in the surgery.
Operative technique
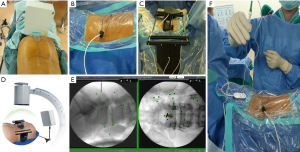
All operations were performed by the same surgical team. The patient was placed in the prone position, and local anesthetic was administered. The magnetic field generator was placed near the surgical site (Figure 2A). After the Kirschner wire was fixed to the spinous process of the adjacent vertebra, the patient localizer was placed on the Kirschner wire and connected to the computer mainframe (Figure 2B). The MaperBrige was placed near the surgical section (Figure 2C). Anterior-posterior and lateral lumbar X-ray were taken by the C-arm, and the images were transmitted to the navigation host for auto-complete registration (Figure 2D,E). The Multipad was then connected to identify and calibrate the different devices (Figure 2F).
The puncture target point was then set up (Figure 3A). An 18-gauge needle was used to access the target point under the guidance of the electromagnetic navigation system (Figure 3B). Once the guidewire reached the target position, the stepwise dilatation guiding rods were inserted to expand the soft tissue, and the protective sheath tube was positioned (Figure 3C). Precise full-visualization foraminoplasty was performed using an endoscope under electromagnetic navigation guidance (Figure 3D). Before introducing the endoscope, the reamer was placed into the protective sheath. Under the endoscope, the soft tissue around the superior articular process (SAP) was removed to clearly reveal the bony structure. The reamer’s resection of the SAP could be observed under the endoscope. The path of the reamer’s advancement and its depth into the spinal canal were monitored in real time by the electromagnetic navigation system until foraminoplasty completion.
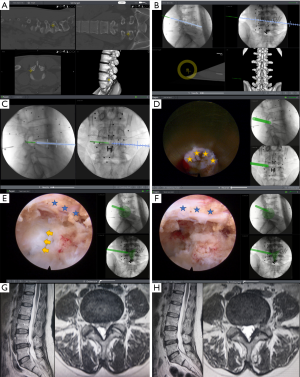
Endoscopic nerve decompression was then performed, and the degenerative tissue in the disc was removed. The nucleus pulposus protruding into the spinal canal was resected first. The fiber annulus was trimmed by using a probe bipolar. The nerve root was confirmed to have been completely released. Under electromagnetic navigation guidance, the real-time position of the endoscope and the range of decompression could be clearly identified on the monitor without any additional X-ray perspective (Figure 3E,F).
Here is one of our cases that a 45-year-old woman with lower back pain and radiating pain in her left lower limb for 2 years, which was aggravated for 3 months. Preoperative magnetic resonance imaging (MRI) indicated L4-5 disc herniation (Figure 3G). She was treated with the I-See electromagnetic-navigation endoscopic spinal surgery system (SEESSYS) and underwent MRI at 6 months after operation (Figure 3H).
Outcome assessment
The overall surgical and fluoroscopic times were recorded for all cases. Outcomes of symptoms were evaluated at 1 day, 2 weeks, 3 months, 6 months, and 12 months, and the last follow-up post-operation. Leg and lower back pain were measured by patients themselves using visual analog scale (VAS) score [1–10]. Functional outcomes were assessed by the Oswestry Disability Index (ODI), and modified MacNab criteria (excellent, good, fair, poor) was measured at last follow up. ODI and modified MacNab criteria were assessed by two doctors. If there was disagreement, a third senior spine doctor was invited to assist in the evaluation to reduce the subjectivity.
Statistical analysis
Statistical analyses were performed with SPSS version 21.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation. The Shapiro-Wilk test was used to check the normality of data. Paired t-test was used for the preoperative and follow-up parameters (VAS and ODI). The descriptive assessments and analytical statistics were performed depending on the group characteristics. Statistical significance was set at P<0.05.
Results
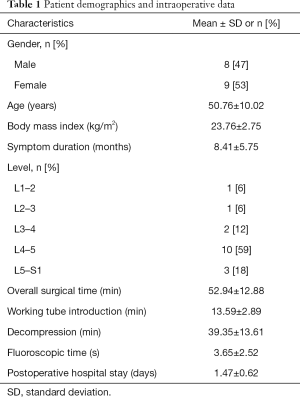
Eighteen LDH patients underwent SEESSYS and one patient was lost to follow-up. Finally, 17 patients were included in this study. The demographic and intraoperative data of the patients are shown in the Table 1. The mean follow-up period was 20.64 months (range, 19–21 months). The average operating time was 52.94±12.88 min (range, 35–78 min), including the working tube introduction time (13.59±2.89 min), decompression time (39.35±13.61 min), and the fluoroscopic time (3.65±2.52 min).
Full table
There was a significant decrease in back VAS, leg VAS, and ODI (P<0.01, P<0.01, and P<0.01, respectively) at 1 day, 2 weeks, 3 months, 6 months, 12 months and the last follow up post-operation compared with pre-operation (Table 2). The back VAS, leg VAS, and ODI decreased significantly from 3.29±0.69, 6.59±0.87, and 63.88±6.91 preoperatively to 0.47±0.52, 0.59±0.71, and 8.94±1.43 at last follow-up (P<0.01), respectively. The overall excellent and good rate was 94% (excellent in 15 patients, good in 1 patient, fair in 1 patient, and no poor patient).

Full table
There were no serious complications, such as nerve and vessel injuries or cerebrospinal fluid leakage. No wound hematomas, infections, and other perioperative complications were found.
Discussion
There are two key issues for completing PELD. One is to place working tube accurately to the target lesion; the other is to relieve the nerve roots completely from compression without new complications. Because of the complex anatomy of the intraspinal canal and the extremely limited room for maneuver, there is a high risk of damage to nerves and blood vessels if not done properly (12).
Some studies have already indicated that, despite the outstanding benefits of this minimally invasive technique, the learning curve of the transforaminal endoscopic surgical system is steep (12-14). For beginners, it is difficult to position the working tube into the spinal canal precisely through the safe triangle. For surgeons who are already proficient in this procedure, it should be performed with the assistance of multiple X-ray photography for optimal safety. Intraoperative X-ray-guided puncture and catheterization is the most commonly used method in current clinical practice. Although it has the benefit of higher image resolution and more accurate localization, CT navigational assistance has the drawback of greater radiation, which has been also reported in previously published studies on endoscopic surgery (15,16). Radiation damage to doctors and patients as a result of X-rays has been gradually gaining attention (17). Mastrangelo et al. reported that there was an increase in tumor development among orthopaedical surgeons exposed to routine radiation (18). Moreover, neither CT- nor X-ray-guided surgical procedure can display the instrument position in real time, and the process of catheterization is a blinded operation, which depends on the skill level of the surgeon. Therefore, some new technologies have been introduced in recent years, such as intraoperative ultrasonic monitoring and guidance (19,20). Although ultrasound navigation is known to reduce radiation injury, its accuracy is still questionable, because ultrasonic perspective is susceptible to bone and air interference. In addition, its blind spot could induce unpredictable noxious stimulation to nerves and blood vessels. Although combining CT and ultrasonographic imaging can provide real-time image data, it has not widely used in the clinical setting.
Previous studies have showed that electromagnetic navigation could reduce surgical complications and minimize surgical time (8,21). Electromagnetic navigation has also been used in the placement of fiducial markers for stereotactic radiotherapy and accurate navigation to target lesions (22). The electromagnetic navigation system generates a magnetic field, which is used to transmit and receive electromagnetic signals to determine the spatial position of the target. The basic principle is to use the magnetic field of known spatial distribution to realize the positioning of objects in the magnetic field according to the data obtained by the sensor (23,24). Through the intraoperative real-time positioning system, the position of the surgical instrument in the field is accurately positioned. The operator can observe the actual position of the instruments by referring to the horizontal, coronal, and sagittal three-dimensional images displayed on the computer monitor (7,8,21-23). Using electromagnetic navigation in percutaneous full-endoscopic surgery, surgeons can observe the farthest safe position that surgical tools can reach at real time, and maximally remove the lesion for a thorough decompression effect. The use of electromagnetic navigation guidance and the I-See (full-visualization) endoscopic system produces highly accurate and safe results. In comparison with optic navigation, electromagnetic navigation reduces fluoroscopic time from X-ray radiation and ease of convenience. It is a useful tool for the surgeon for precise catheterization, faster decompression, and safety.
Although there were no complications in this study, there was still the possibility of complications if the operation was improper. The potential complications of SEESSYS are the same as those of traditional percutaneous endoscopic lumbar foraminoplasty and discectomy including vascular injury, nerve root injury, dural tear, incomplete decompression, postoperative infection, hematoma and other complications (25). The potential clinical application risk is that the navigation may be not accurate if the localizer moves or its sensitivity decreases. In order to reduce risks, the localizer is fixed to the spinous process of the adjacent vertebra to increase the stability and fluoroscopy can be performed to verify its accuracy, especially for beginners. Once the deviation is found, it should be re-registered immediately. It is also important to note electromagnetic navigation system can be affected if the metal in the patient is ferromagnetic. But titanium alloy, the most commonly used materials for biomedical applications, is nonmagnetic and does not affect electromagnetic navigation system. If the metal material is not clear, the sensor can be placed near the metal and it will display whether the electromagnetic navigation system is affected. When there is an interference, electromagnetic navigation should not be used. The main limitations of this feasibility study are its small sample size and potential selection bias. However, the influence from selective bias in the study is possibly limited because a high participation rate of 94% (17/18 patients) was attained. Therefore, future larger-scale studies with long-term follow-up are needed to corroborate these findings and extend them to a wider population.
Conclusions
Electromagnetic navigation provides accurate guidance for percutaneous spinal endoscopy, displaying the anatomical position of the surgical instrument in real time. This technique could help the spinal surgeon maximally remove the lesion for a thorough decompression effect, and possibly reduce the occurrence of surgical complications, which has great clinical application value. Moreover, this system can reduce fluoroscopic time to doctors and patients. At present, the results with this technique are still preliminary, but are encouraging. We believe that the combination use of the electromagnetic navigation system and endoscopic surgery will further develop the accuracy, safety, and efficiency of percutaneous endoscopic surgery.
Acknowledgments
Funding: This work was partially supported by a grant from the Guangdong Provincial Medical Research Fund (No. 2017460 for Y Lin) and the Second Batch of Dominant Diseases (lumbar disc herniation) in Guangdong Provincial Hospital of Chinese Medicine (No. Y0080).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1956
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1956
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1956). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committees of Guangdong Provincial Hospital of Chinese Medicine (approval No. ZE2019-285-01). Written informed consent was obtained from participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Augoulea A, Tsakonas E, Triantafyllopoulos I, et al. Comparative effects of denosumab or bisphosphonate treatment on bone mineral density and calcium metabolism in postmenopausal women. J Musculoskelet Neuronal Interact 2017;17:444-9. [PubMed]
- Kim HS, Paudel B, Jang JS, et al. Percutaneous Endoscopic Lumbar Discectomy for All Types of Lumbar Disc Herniations (LDH) Including Severely Difficult and Extremely Difficult LDH Cases. Pain Physician 2018;21:E401-8. [PubMed]
- Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol 2005;17:641-61. [Crossref] [PubMed]
- Yeung AT. Minimally Invasive Disc Surgery with the Yeung Endoscopic Spine System (YESS). Surg Technol Int 1999;8:267-77. [PubMed]
- Gibson JN, Cowie JG, Iprenburg M. Transforaminal endoscopic spinal surgery: the future 'gold standard' for discectomy? - A review. Surgeon 2012;10:290-6. [Crossref] [PubMed]
- Morrison JC, Van Batavia JP, Darge K, et al. Ultrasound guided ureteroscopy in children: Safety and success. J Pediatr Urol 2018;14:64.e1-64.e6. [Crossref] [PubMed]
- Liby P, Zamecnik J, Kyncl M, et al. Electromagnetic navigation-guided neuroendoscopic removal of radiation-induced intraforniceal cavernoma as a late complication of medulloblastoma treatment. Childs Nerv Syst 2017;33:2051-5. [Crossref] [PubMed]
- Chang CM, Jaw FS, Lo WC, et al. Three-dimensional analysis of the accuracy of optic and electromagnetic navigation systems using surface registration in live endoscopic sinus surgery. Rhinology 2016;54:88-94. [Crossref] [PubMed]
- Berger M, Nova I, Kallus S, et al. Electromagnetic navigated condylar positioning after high oblique sagittal split osteotomy of the mandible: a guided method to attain pristine temporomandibular joint conditions. Oral Surg Oral Med Oral Pathol Oral Radiol 2018;125:407-14.e1. [Crossref] [PubMed]
- von Jako RA, Carrino JA, Yonemura KS, et al. Electromagnetic navigation for percutaneous guide-wire insertion: Accuracy and efficiency compared to conventional fluoroscopic guidance. Neuroimage 2009;47:T127-32. [Crossref] [PubMed]
- Sagi HC, Manos R, Benz R, et al. Electromagnetic field-based image-guided spine surgery part one: Results of a cadaveric study evaluating lumbar pedicle screw placement. Spine 2003;28:2013-8. [Crossref] [PubMed]
- Zhou C, Zhang G, Panchal RR, et al. Unique Complications of Percutaneous Endoscopic Lumbar Discectomy and Percutaneous Endoscopic Interlaminar Discectomy. Pain Physician 2018;21:E105-12. [PubMed]
- Ao S, Wu J, Tang Y, et al. Percutaneous Endoscopic Lumbar Discectomy Assisted by O-Arm-Based Navigation Improves the Learning Curve. Biomed Res Int 2019;2019:6509409. [Crossref] [PubMed]
- Lee DY, Lee SH. Learning curve for percutaneous endoscopic lumbar discectomy. Neurol Med Chir (Tokyo) 2008;48:383-8; discussion 388-9. [Crossref] [PubMed]
- Oyelese AA, Fridley J, Choi DB, et al. Minimally invasive direct lateral, retroperitoneal transforaminal approach for large L1-2 disc herniations with intraoperative CT navigational assistance: technical note and report of 3 cases. J Neurosurg Spine 2018;29:46-53. [Crossref] [PubMed]
- Oyelese A, Telfeian AE, Gokaslan ZL, et al. Intraoperative Computed Tomography Navigational Assistance for Transforaminal Endoscopic Decompression of Heterotopic Foraminal Bone Formation After Oblique Lumbar Interbody Fusion. World Neurosurg 2018;115:29-34. [Crossref] [PubMed]
- Narain AS, Hijji FY, Yom KH, et al. Radiation exposure and reduction in the operating room: Perspectives and future directions in spine surgery. World J Orthop 2017;8:524-30. [Crossref] [PubMed]
- Mastrangelo G, Fedeli U, Fadda E, et al. Increased cancer risk among surgeons in an orthopaedic hospital. Occup Med (Lond) 2005;55:498-500. [Crossref] [PubMed]
- Wu R, Liao X, Xia H. Radiation Exposure to the Surgeon During Ultrasound-Assisted Transforaminal Percutaneous Endoscopic Lumbar Discectomy: A Prospective Study. World Neurosurg 2017;101:658-65.e1. [Crossref] [PubMed]
- Liu YB, Wang Y, Chen ZQ, et al. Volume Navigation with Fusion of Real-Time Ultrasound and CT Images to Guide Posterolateral Transforaminal Puncture in Percutaneous Endoscopic Lumbar Discectomy. Pain Physician 2018;21:E265-78. [PubMed]
- Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: A descriptive analysis. J Thorac Dis 2012;4:173-85. [PubMed]
- Vieira T, Stern JB, Girard P, et al. Endobronchial treatment of peripheral tumors: ongoing development and perspectives. J Thorac Dis 2018;10:S1163-7. [Crossref] [PubMed]
- Sánchez Y, Trifanov DS, Kattapuram TM, et al. Use of an Electromagnetic Navigation System on a Phantom as a Training Simulator for CT-Guided Procedures. J Am Coll Radiol 2017;14:795-9. [Crossref] [PubMed]
- Usuda J. Virtual Bronchoscopic Navigation (VBN) and Electromagnetic Navigation System. Kyobu Geka 2018;71:843-9. [PubMed]
- Yin J, Jiang Y, Nong L. Transforaminal approach versus interlaminar approach: A meta-analysis of operative complication of percutaneous endoscopic lumbar discectomy. Medicine (Baltimore) 2020;99:e20709. [Crossref] [PubMed]
(English Language Editor: R. Scott)


