Clinicopathological and prognostic value of circulating tumor cells in esophageal carcinoma: a meta-analysis
Introduction
Esophageal cancer (EC) is the eighth most common malignant tumor worldwide and the sixth leading cause of cancer-related death worldwide, with approximately 572,000 new cases and 509,000 deaths (1). There are two most common histopathological subtypes, squamous cell carcinoma and adenocarcinoma. Patients with EC, compared with those with other cancers, have poorer prognosis because of earlier metastasis or recurrence (2). Although new strategies including preoperative radio chemotherapy and three-field lymph node dissection have been implemented, the outcomes are still unsatisfactory. Therefore, biomarkers that can identify the recurrence or metastasis are needed to facilitate timely diagnosis and treatment strategies and thus improve the prognosis of EC patients.
Circulating tumor cells (CTCs), which are defined as cancer cells that have escaped from the primary tumor into the circulation, have great promise as a “liquid biopsy”, a noninvasive method of assessing tumor progression in real time. CTCs have several advantages over other technologies. Firstly, it is directly related to invasion and development of metastasis, and can even reflect the micrometastases status to a certain degree (3). Secondly, the collection of peripheral blood samples is convenient and simple, without radioactive pollution or risk of massive hemorrhage.
New research shows that a fraction of CTCs is capable of entering distant sites and progressing toward metastases. CTCs can remain non-proliferative state for a long period of time and resist the anti-tumor effect of chemotherapy drugs (4). In fact, the significance of CTCs in the peripheral blood of patients with various malignancies has been widely studied extensively in various malignancies. Previous studies have demonstrated that CTCs are closely related to tumor prognosis in breast cancer (5), lung cancer (6), gastric cancer (7), and pancreatic cancer (8). In the past two decades, a considerable amount of literature studied the relationship between CTCs and EC. Two previous meta-analysis (9,10) found that CTC status was related to TNM stage of cancer, but was not related to T stage or degree of differentiation in patients in esophageal patients. No significant relationship was found between CTCs and survival time of EC, definitely. Wang conducted a meta-analysis (11) providing strong evidence that detection of CTCs in the peripheral blood was an independent prognostic indicator of poor outcome for esophageal squamous cell carcinoma (ESCC) patients. Whereas Wang only based only on 13 studies and 715 patients, without specifying the correlation between clinicopathological parameters and CTCs status. Our meta-analysis will be the first systematic review to clearly investigate this issue and evaluate potential sources of heterogeneity that may affect some existing conclusions. We presented the following article in accordance with the PRISMA Checklist (available at http://dx.doi.org/10.21037/apm-20-590).
Methods
Search strategy
This systematic review and meta-analysis were registered at International Prospective Register of Systematic Reviews (CRD42019125600). PubMed, EMBASE, ISI Web of Science database and Cochrane Library were searched for eligible studies between January, 2000 and August, 2019. The following search terms were used for the literature search: (circulating tumor cells OR circulating cancer cells OR CTCs) AND (esophageal carcinoma OR esophageal cancer OR oesophageal cancer). The last search was conducted on January 17, 2020. The language was limited to English. Two authors independently retrieved the titles and abstracts of the primary studies identified in the electronic search. In addition, references of potentially relevant studies were also examined.
Inclusion and exclusion criteria
Articles, which met the following criteria, were included in the meta-analysis: (I) investigated the clinicopathological or prognostic of CTC detection in EC patients; (II) reported hazard ratio (HR) or a risk ratio (RR) with a 95% confidence interval (CI) of overall survival (OS) or/and progression-free survival (PFS) in the study or had sufficient data to calculate a RR of clinicopathological characteristic; (III) collected samples from PB; (IV) the study with observational design. Exclusion criteria were: (I) review articles, letters, comments and case reports; and (II) studies unable to retrieve or calculate data of interest. To avoid the inclusion of duplicated studies, all the included studies were carefully checked, including their authors, organization, accrual periods, and population of patients.
Data extraction and quality assessment
Two reviewers independently examined the included studies for eligibility and retrieved the information from all eligible studies. The following information was collected: first author, year of publication, country, characteristics of the study population (number, sex and age), TNM stage, adjuvant therapy, detection marker, CTCs-positive rate, treatment, follow-up period, the HR and its associated standard errors on prognostic outcomes (OS or/and PFS). If the HR and its 95% CI were not directly provided in the original articles, the method used was to incorporate summary time-to-event data into meta-analysis (12). In addition, if available, multivariate analysis was preferable because it also considered possible confounding factors (13). The methodological quality was assessed by two authors using the Newcastle-Ottawa Scale (NOS) with 0-3 scores defined as low quality, 4-6 scores as moderate quality, and 7-9 scores as high quality (14,15). Discrepancies between the two reviewers were resolved through discussion and consensus. If still no agreement was reached, an additional adjudicator was invited into the discussion.
Statistical analysis
HR, RR and their associated 95% CI were used as the effect indicators for summarizing the clinicopathological and prognostic significance of CTCs in EC. If available, multivariate-adjust risks were used for each study. All eligible studies were included in the analysis. The heterogeneity between studies was evaluated with Q and I2 statistics (16). Studies with an I2 statistics of 0%, 25%, 50% and 75% represented no, low, moderate, and high heterogeneity. According to the results of inter-study heterogeneity appraisal using Q and I2 statistics, pooled RRs and HRs with 95% CI were calculated using a fixed-effect model (Mantel-Haenszel method) or random-effect model (DerSimonian-Laird method) (17). Sensitivity analysis was performed to assess the impact of a single study on the meta-analysis estimated by sequential omission of individual studies. If necessary, the heterogeneity was also explored by subgroup and meta-regression. The potential publication bias was further validated by the Egger’s and Begg’s test (18). The STATA version 12.0 (Stata Corp LP, College Station, Texas, USA) was used for statistical analysis. All statistical analyses were two sides. A P value less than 0.05 was considered statistically significant. The data-analysis started in February 10, 2020 and was completed in February 14, 2020.
Results
Study selection and characteristics
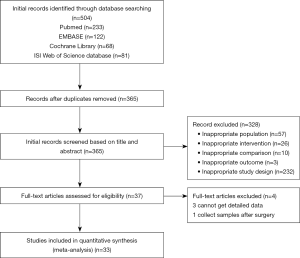
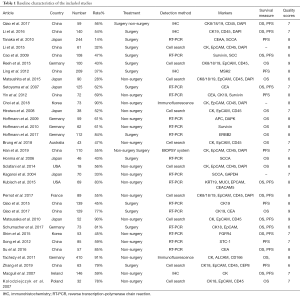
The PRISMA flow chart of this meta-analysis was shown in Figure 1. Duplicates and irrelevant studies or those without sufficient data were removed from a total of 504 publications. All investigators finally agreed to include 33 eligible studies in our meta-analysis (Table 1). Among these, Seventeen studies (19-35) were conducted on esophageal squamous cell carcinoma (ESCC), and nine (36-44) addressed esophageal adenocarcinoma cancer (EAC). Other studies (45-51) addressed both. Three (22,28,29) only reported Clinicopathological parameters. The average age (median or mean) in the included studies was ranged from 58.9 to 65 years. The sample size was ranged from 18 to 410. All studies were of moderate or high quality.
Full table
Correlation between CTCs and OS
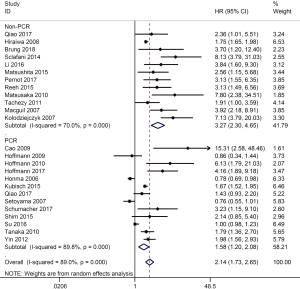
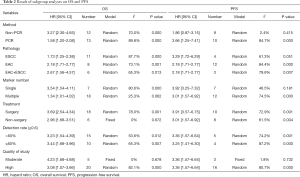
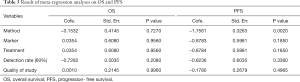
Data on OS were available in 25 studies (19-21,23,25-27,31,32,34,36-48,50,51). With considerable evidence of heterogeneity between studies (I2=89.0%, P=0.000), the data from the subgroups within a single study was pooled using a random-effect model. The pooled results showed that OS of patients with CTCs-positive EC was significantly lower than that of CTCs-negative patients (HR =2.14; 95% CI, 1.73–2.65; Figure 2). We performed subgroup analysis to further assess whether the CTC positivity had prognostic value in different subsets (Table 2), and the stratified results showed that compared with CTCs-negative patients, CTCs-positive patients had a higher risk for poor OS in these subgroups. As to the difference of the detection methods, especially, the studies were divided into two subgroups (the PCR group and the non-PCR group). A significant difference in OS between CTC-positive and CTC-negative patients was found in both PCR and Non-PCR subgroups. The estimated HR was 3.27 (95% CI, 2.30–4.65) in the PCR subgroup and 1.58 (95% CI, 1.20–2.08) in the non-PCR subgroup. The meta-regression analysis showed no significant role of a variable to account for the heterogeneity (Table 3) and no single study markedly changed the overall effect on the sensitivity analysis.
Full table
Full table
Correlation between CTCs and PFS
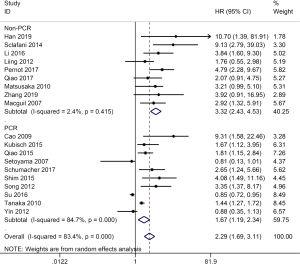
Nineteen studies (19-21,23,24,26,27,30-35,39,40,43,44,49,51) were included in this meta-analysis. High heterogeneity was shown among the studies (I2=83.4%, P=0.000). Therefore, the data was pooled in a random-effect. The pooled data revealed that compared with CTCs-negative EC patients, the CTCs-positive patients had a higher risk of disease progression (HR =2.29; 95% CI, 1.69–3.11, Figure 3). The meta-regression was further performed to explore the source of heterogeneity on PFS. As showed in Table 3, only the method of CTC detection was significantly correlated with intra-study variability (P=0.021), which explained 92.42% of the heterogeneity in the analysis. Furthermore, we conducted subgroup analysis to evaluate the prognostic value of CTCs detected by two most common methods respectively. The pooled HR, in random-effect, for the studies based on RT-PCR that assessed the association between CTCs and the PFS of EC (HR =1.67; 95% CI, 1.19–2.34, Figure 3), with high heterogeneity (I2=84.7%, P=0.000). However, when studies on non-PCR were combined, no high heterogeneity was found (I2=2.4%, P=0.415), with high HR (HR =3.32; 95% CI, 2.43–3.53, Figure 3) in fixed-effect. In other subgroup analysis, the overall effect did not change significantly in the subgroup. In the sensitivity analysis, the exclusion of any single study did not remarkably change the overall effect.
Association between CTCs and clinicopathological parameters
Thirteen studies including 15 sets of data were evaluated to determine the relationship between CTC-positive and TNM stage. With moderate heterogeneity (I2=59.8%, P<0.05), the results showed, TNM stage was associated with CTC positivity (RR =1.36; 95% CI, 1.09–1.69, P=0.22). The depth of tumor infiltration was associated with the CTC positivity (RR =1.42; 95% CI, 1.10–1.82, P=0.21), with low heterogeneity (I2=48.0%, P=0.027), but the regional lymph nodes metastasis was not statistically associated with the CTC positivity (RR =1.31; 95% CI, 0.96–1.80, P=0.76), with high heterogeneity (I2=75.4%, P<0.05). Studies assessed by pooled analyses showed no significant association between CTC-positive and distant metastasis (RR =1.58; 95% CI, 1.00–2.50, P=0.65), with high heterogeneity (I2=84.4%, P=0.000). Similarly, the data from eight studies demonstrated that tumor grade was not associated with the CTC positivity (RR =0.88; 95% CI, 0.71–1.09, P=0.15). Low heterogeneity was shown among studies (I2=29.2%, P=0.195). And all analyses were conducted in random-effect. In addition, the EC adjusted survival rates did not differ by anatomic location of the tumor. Moreover, sensitivity analysis confirmed that no individual study influenced affected the overall results.
Publication bias
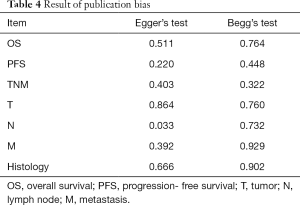
The publication bias in this meta-analysis were indicated by Egger’s test and Begg’s test. The results were shown in Table 4. Notably, a significant publication bias was revealed by Egger’s test (P=0.033) on the association between regional lymph nodes metastasis and CTC-positive, but not by the Begg’s test (P=0.732). The conclusions were not changed after adjustment for publication bias by using the trim and fill method (52).
Full table
Discussion
In this meta-analysis, we discussed the prognostic value of CTCs in EC, including 3,271 patients with 33 publications. Survival outcome could be obtained from 31 studies including 3,073 patients. The pooled data demonstrated that CTC-positive patients had poorer OS and DFS than CTC-negative patients, suggesting that CTCs is a useful biomarker for the clinical prognosis of patients with EC. In addition, this meta-analysis assessed the correlation between clinicopathological parameters of EC patients and the results showed that the depth of tumor and later TNM stage were significantly correlated with CTC positivity.
A meta-analysis discussed clinicopathological and prognostic value of CTC-positive patients for both PFS and OS in patients with EC. However, due to the significant heterogeneity in PFS, they only confirmed the clinical value for OS (10).
Therefore, this meta-analysis took peripheral blood samples of patients with EC and analyzed the clinicopathological and prognostic value of CTCs. We included high quality studies with sufficiently large sample. At present, the prognosis outcome of EC patients is still guided by the TNM stage, which is influenced by clinicopathological parameters such as vascular invasion, poor differentiation, tumor size and serum tumor markers. In terms of cost and simple operation, CTC analysis has the advantages to serve as a monitoring tool pre- or/and post-treatment. Several studies suggested that CTCs detection could provide important prognostic information for patients with EC (53). Many factors may influence the CTCs status. The sampling time, pre- or post-treatment, also seemed to play an important role in CTC analysis. In endometrial cancer, the relationship between prognosis and post-treatment CTC status was more convincing because post-treatment CTCs status contained pre-treatment CTCs and released CTCs during therapy, especially operation (54). However, rapid apoptotic death of pre-treatment CTCs may release massive tumor genes or antigens due to the change of the survival microenvironment in the process of operation, which might cause detection bias. Hence, uncertainties still remained, and the sampling time could provide more prognostic information, which needs further research work to confirm this relationship.
Several limitations of this study must be acknowledged. First, although several subgroup analyses were performed, significant heterogeneity was generally observed. Given the differences of the studies in age, subjects’ lifestyle, information collection method, sample size and so on, the heterogeneity was inevitable. We addressed the heterogeneity by using a random effects model to obtain a more conservative result. Second, the number of stratified analysis was so limited that might cause a result in invalid statistical analyses in those groups. Our overall results lead to imprecision in the results. Besides, several sources of bias would be crude. Third, although we used multivariate statistical models to calculate the estimated RR, the number and content of the adjusted confounders varied in each trial, as inherent limitations, unmeasured confounding, and the typical bias in observational studies, may influence the observed results.
Our meta-analysis systematically assessed the prognostic significance of CTCs in EC patients. Our results suggested that standardized testing method, optimized sampling time, complete analysis and report of results played an important role in deriving more accurate prognostic significance of CTCs in EC patients.
Conclusions
Our meta-analysis suggested the CTCs testing has high prognostic value in EC and confirmed that CTC-positive patients were associated with poor PFS and OS. In addition, we found that CTC-positive patients were significantly associated with depth of infiltration and clinical pathologic staging. However, CTCs status was less supportive as an indicator of the risk of more lymph node metastasis or distant organ metastasis. Further studies are warranted to address these issues as an attempt to bring CTCs from the lab bench to the hospital bedside in EC.
Acknowledgments
The authors would like to thank Yan Li for her assistance with the article searches.
Funding: This study was supported by the Key Medical Research Fund of Hebei Provincial Health Planning Commission (20160177 and 20180528).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-590
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-590). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen J, Cai W, Lin Y, et al. Patterns and rates of abdominal lymphatic metastasis following esophageal carcinoma. PLoS One 2017;12:e0185424. [Crossref] [PubMed]
- Andreou A, Schmelzle M, Sauer IM, et al. The Impact of Tumor Cell Proliferation on Occult Micrometastases, Tumor Recurrence and Patient Outcome Following Resection for Liver Malignancies. Zentralbl Chir 2016;141:375-82. [PubMed]
- Soave A, Riethdorf S, Pantel K, et al. Do circulating tumor cells have a role in deciding on adjuvant chemotherapy after radical cystectomy? Curr Urol Rep 2015;16:46. [Crossref] [PubMed]
- Zhang L, Ridgway LD, Wetzel MD, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med 2013;5:180ra48. [Crossref] [PubMed]
- Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol 2017;7:170070. [Crossref] [PubMed]
- Watanabe T, Okumura T, Hirano K, et al. Circulating tumor cells expressing cancer stem cell marker CD44 as a diagnostic biomarker in patients with gastric cancer. Oncol Lett 2017;13:281-8. [Crossref] [PubMed]
- Poruk KE, Blackford AL, Weiss MJ, et al. Circulating Tumor Cells Expressing Markers of Tumor-Initiating Cells Predict Poor Survival and Cancer Recurrence in Patients with Pancreatic Ductal Adenocarcinoma. Clin Cancer Res 2017;23:2681-90. [Crossref] [PubMed]
- Xu HT, Miao J, Liu JW, et al. Prognostic value of circulating tumor cells in esophageal cancer. World J Gastroenterol 2017;23:1310-8. [Crossref] [PubMed]
- Hou J, Zou K, Yang C, et al. Clinicopathological and prognostic significance of circulating tumor cells in patients with esophageal cancer: a meta-analysis. Onco Targets Ther 2018;11:8053-61. [Crossref] [PubMed]
- Wang S, Du H, Li G. Significant prognostic value of circulating tumor cells in esophageal cancer patients: A meta-analysis. Oncotarget 2017;8:15815-26. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013;13:152. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195. [Crossref] [PubMed]
- Takkouche B, Khudyakov P, Costa-Bouzas J, et al. Confidence intervals for heterogeneity measures in meta-analysis. Am J Epidemiol 2013;178:993-1004. [Crossref] [PubMed]
- Schmidt FL. History and development of the Schmidt-Hunter meta-analysis methods. Res Synth Methods 2015;6:232-9. [Crossref] [PubMed]
- Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018;74:785-94. [Crossref] [PubMed]
- Qiao Y, Li J, Shi C, et al. Prognostic value of circulating tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Onco Targets Ther 2017;10:1363-73. [Crossref] [PubMed]
- Li SP, Guan QL, Zhao D, et al. Detection of Circulating Tumor Cells by Fluorescent Immunohistochemistry in Patients with Esophageal Squamous Cell Carcinoma: Potential Clinical Applications. Med Sci Monit 2016;22:1654-62. [Crossref] [PubMed]
- Tanaka K, Yano M, Motoori M, et al. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol 2010;17:2779-86. [Crossref] [PubMed]
- Li H, Song P, Zou B, et al. Circulating Tumor Cell Analyses in Patients With Esophageal Squamous Cell Carcinoma Using Epithelial Marker-Dependent and -Independent Approaches. Medicine (Baltimore) 2015;94:e1565. [Crossref] [PubMed]
- Cao M, Yie SM, Wu SM, et al. Detection of survivin-expressing circulating cancer cells in the peripheral blood of patients with esophageal squamous cell carcinoma and its clinical significance. Clin Exp Metastasis 2009;26:751-8. [Crossref] [PubMed]
- Ling ZQ, Zhao Q, Zhou SL, et al. MSH2 promoter hypermethylation in circulating tumor DNA is a valuable predictor of disease-free survival for patients with esophageal squamous cell carcinoma. Eur J Surg Oncol 2012;38:326-32. [Crossref] [PubMed]
- Matsushita D, Uenosono Y, Arigami T, et al. Clinical Significance of Circulating Tumor Cells in Peripheral Blood of Patients with Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2015;22:3674-80. [Crossref] [PubMed]
- Setoyama T, Natsugoe S, Okumura H, et al. Isolated tumour cells in blood and E-cadherin expression in oesophageal squamous cell cancer. Br J Surg 2007;94:984-91. [Crossref] [PubMed]
- Yin XD, Yuan X, Xue JJ, et al. Clinical significance of carcinoembryonic antigen-, cytokeratin 19-, or survivin-positive circulating tumor cells in the peripheral blood of esophageal squamous cell carcinoma patients treated with radiotherapy. Dis Esophagus 2012;25:750-6. [Crossref] [PubMed]
- Choi MK, Kim GH. Circulating tumor cells detected using fluid-assisted separation technique in esophageal squamous cell carcinoma. J Gastroenterol Hepatol 2019;34:552-60. [Crossref] [PubMed]
- Kaganoi J, Shimada Y, Kano M, et al. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg 2004;91:1055-60. [Crossref] [PubMed]
- Qiao YF, Chen CG, Yue J, et al. Clinical significance of preoperative and postoperative cytokeratin 19 messenger RNA level in peripheral blood of esophageal cancer patients. Dis Esophagus 2016;29:929-36. [Crossref] [PubMed]
- Matsusaka S, Chin K, Ogura M, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci 2010;101:1067-71. [Crossref] [PubMed]
- Shim HJ, Shin MH, Kim HN, et al. The Prognostic Significance of FGFR4 Gly388 Polymorphism in Esophageal Squamous Cell Carcinoma after Concurrent Chemoradiotherapy. Cancer Res Treat 2016;48:71-9. [Crossref] [PubMed]
- Song H, Xu B, Yi J. Clinical significance of stanniocalcin-1 detected in peripheral blood and bone marrow of esophageal squamous cell carcinoma patients. J Exp Clin Cancer Res 2012;31:35. [Crossref] [PubMed]
- Su PJ, Wu MH, Wang HM, et al. Circulating Tumour Cells as an Independent Prognostic Factor in Patients with Advanced Oesophageal Squamous Cell Carcinoma Undergoing Chemoradiotherapy. Sci Rep 2016;6:31423. [Crossref] [PubMed]
- Zhang Y, Li J, Wang L, et al. Clinical significance of detecting circulating tumor cells in patients with esophageal squamous cell carcinoma by EpCAM-independent enrichment and immunostaining-fluorescence in situ hybridization. Mol Med Rep 2019;20:1551-60. [PubMed]
- Hiraiwa K, Takeuchi H, Hasegawa H, et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol 2008;15:3092-100. [Crossref] [PubMed]
- Brungs D, Lynch D, Luk AW, et al. Cryopreservation for delayed circulating tumor cell isolation is a valid strategy for prognostic association of circulating tumor cells in gastroesophageal cancer. World J Gastroenterol 2018;24:810-8. [Crossref] [PubMed]
- Honma H, Kanda T, Ito H, et al. Squamous cell carcinoma-antigen messenger RNA level in peripheral blood predicts recurrence after resection in patients with esophageal squamous cell carcinoma. Surgery 2006;139:678-85. [Crossref] [PubMed]
- Kubisch I, de Albuquerque A, Schuppan D, et al. Prognostic Role of a Multimarker Analysis of Circulating Tumor Cells in Advanced Gastric and Gastroesophageal Adenocarcinomas. Oncology 2015;89:294-303. [Crossref] [PubMed]
- Pernot S, Badoual C, Terme M, et al. Dynamic evaluation of circulating tumour cells in patients with advanced gastric and oesogastric junction adenocarcinoma: Prognostic value and early assessment of therapeutic effects. Eur J Cancer 2017;79:15-22. [Crossref] [PubMed]
- Qiao YF, Chen CG, Yue J, et al. Prognostic significance of preoperative and postoperative CK19 and CEA mRNA levels in peripheral blood of patients with gastric cardia cancer. World J Gastroenterol 2017;23:1424-33. [Crossref] [PubMed]
- Kolodziejczyk P, Pituch-Noworolska A, Drabik G, et al. The effects of preoperative chemotherapy on isolated tumour cells in the blood and bone marrow of gastric cancer patients. Br J Cancer 2007;97:589-92. [Crossref] [PubMed]
- Sclafani F, Smyth E, Cunningham D, et al. A pilot study assessing the incidence and clinical significance of circulating tumor cells in esophagogastric cancers. Clin Colorectal Cancer 2014;13:94-9. [Crossref] [PubMed]
- Schumacher S, Bartenhagen C, Hoffmann M, et al. Disseminated tumour cells with highly aberrant genomes are linked to poor prognosis in operable oesophageal adenocarcinoma. Br J Cancer 2017;117:725-33. [Crossref] [PubMed]
- Reeh M, Effenberger KE, Koenig AM, et al. Circulating Tumor Cells as a Biomarker for Preoperative Prognostic Staging in Patients With Esophageal Cancer. Ann Surg 2015;261:1124-30. [Crossref] [PubMed]
- Hoffmann AC, Vallbohmer D, Prenzel K, et al. Methylated DAPK and APC promoter DNA detection in peripheral blood is significantly associated with apparent residual tumor and outcome. J Cancer Res Clin Oncol 2009;135:1231-7. [Crossref] [PubMed]
- Hoffmann AC, Vallbohmer D, Grimminger P, et al. Preoperative survivin mRNA detection in peripheral blood is an independent predictor of outcome in esophageal carcinoma. Pharmacogenomics 2010;11:341-7. [Crossref] [PubMed]
- Hoffmann M, Pasch S, Schamberger T, et al. Diagnostic pathology of early systemic cancer: ERBB2 gene amplification in single disseminated cancer cells determines patient survival in operable esophageal cancer. Int J Cancer 2018;142:833-43. [Crossref] [PubMed]
- Han L, Li YJ, Zhang WD, et al. Clinical significance of tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2019;98:e13921. [Crossref] [PubMed]
- Tachezy M, Effenberger K, Zander H, et al. ALCAM (CD166) expression and serum levels are markers for poor survival of esophageal cancer patients. Int J Cancer 2012;131:396-405. [Crossref] [PubMed]
- MacGuill MJ, Barrett C, Ravi N, et al. Isolated tumour cells in pathological node-negative lymph nodes adversely affect prognosis in cancer of the oesophagus or oesophagogastric junction. J Clin Pathol 2007;60:1108-11. [Crossref] [PubMed]
- Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Based Ment Health 2014;17:30. [Crossref] [PubMed]
- Court CM, Ankeny JS, Sho S, et al. Circulating Tumor Cells in Gastrointestinal Cancer: Current Practices and Future Directions. Cancer Treat Res 2016;168:345-76. [Crossref] [PubMed]
- Ezewuiro O, Grushko TA, Kocherginsky M, et al. Association of Metformin Use with Outcomes in Advanced Endometrial Cancer Treated with Chemotherapy. PLoS One 2016;11:e0147145. [Crossref] [PubMed]