
The anti-CXCL4 antibody depletes CD4(+)CD25(+)FOXP3(+) regulatory T cells in CD4+ T cells from chronic osteomyelitis patients by the STAT5 pathway
Introduction
Osteomyelitis covers a large pattern of inflammatory bone disorders caused by infectious organisms or auto-inflammatory processes (1). Currently, posttraumatic or postoperative osteomyelitis, the main subtype of osteomyelitis, stands for 80% of a chronic state of osteomyelitis due to sustaining bacterial biofilm from foreign materials (1,2). Immune suppression frequently occurs among chronic osteomyelitis (3). Noteworthy, osteonecrosis and bone resorption regarding infectious organisms usually disturb antibiotics treatments of this disease, thus subscribe the bacterial with immune evasion (4). Simultaneously, the immune-regulatory treatment might provide a therapeutic possibility of chronic osteomyelitis (5). Hence, the therapeutic immune modulation or exploring the underlying mechanisms has captured an increased clinical attention to block the chronic osteomyelitis.
CD4(+)CD25(+)FOXP3(+) regulatory T cells (Tregs), marked by the expression of forkhead box P3 (FOXP3), are a specialized subset of CD4+ T cells, accounting for 5–10% of peripheral CD4+ T cells. Tregs exert an immune-suppressive property and are usually up-regulated in chronic inflammatory disease (6). Mechanically, Tregs function in inhibiting potentially auto-reactive T cells, maintaining immune tolerance and controlling immune responses during allergy, autoimmunity, inflammation as well as tumors immunity, which render it the core component of peripheral tolerance (7-10). Therefore, dysfunction or downregulation of Tregs might represent an immunotherapy strategy to restrict chronic osteomyelitis (11).
Tregs express significant amounts of C-X-C chemokine family members that mediate the traffic and recruitment of immune cells to the inflammation sites (12). CXC family member CXCL10 and CXCL14 are documented important roles in immune regulation in inflammatory-associated coronary artery disease, atherosclerosis (13,14), or ischemic stroke (15). CXCL4 is involved in atherosclerosis and other inflammatory diseases through forming a CC-type heterodimer with CCL5 (16), drives chemotaxis of CCR1-expressing cells (17) and its neutralizing antibody activates platelets in autoimmune disorders, especially in thrombocytopenia in the absence of heparin (18). Report has demonstrated that CXCL4 augment the secretion of pro-inflammatory cytokines of human CD4+ T cells, and meanwhile favors the stimulation of T1 and T17 cells, the two subsets of CD4+ T cells, in psoriatic arthritis (19). However, whether CXCL4 regulated Tregs and whether CXCL4 would represent an immunomodulator in chronic osteomyelitis remained largely unknown.
Signal transducer and activator of transcription 5 (STAT5) plays a key role in the development of Tregs (20). CXCL4 signaling experiments show involvement of mitogen-activated protein (MAP) kinases, Src and p70S6 kinase in cells expressing CXCR3A and CXCR3B (21). Evidence suggests that chemokine CXCL12 activates STAT5 pathway (22). In this work, to study the effect of chemokine CXCL4 on Tregs induction, and whether STAT5 pathway was the underlying mechanism, CD4+ T cells, isolated from patients with chronic osteomyelitis and corresponding healthy donor (HD), were treated with anti-CXCL4 antibody or recombinant CXCL4 protein in vitro. STAT5 inhibitor (IST5-002) was used to block STAT5 pathway (23). Our data suggested that CXCL4 regulated the percentage of Tregs in CD4+ T cells via JAK3/STAT5 pathway.
Methods
Enzyme-linked immunosorbent assay (ELISA) assay
To study the involvement of CXCL4 in chronic osteomyelitis, serum sample of 37 patients with chronic osteomyelitis and 30 corresponding healthy people were recruited from Tongde Hospital of Zhejiang Province without significant difference in age and gender between those two groups. The inclusion criteria were as follows: (I) diagnosis with chronic osteomyelitis; (II) age: 18–70; (III) with the patient’s informed consent. And the exclusion criteria were as follows: (I) with diseases of heart, liver, kidney and other organs; (II) with diabetes; (III) with other infectious diseases; (IV) with bone tuberculosis and tumor. And the patient demographic was listed in Table 1. This study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province (No. 2016-016), and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent from the participants was obtained. Human platelet factor 4 (PF4/CXCL4) ELISA assay kit (ab189573, Abcam, USA) was used to determine serum CXCL4 concentration (ng/mL) accordance with the manufacture’s procedure.

Full table
Preparation of peripheral blood mononuclear cells (PBMCs)
Peripheral bloods (30 mL) from 37 chronic osteomyelitis cases and 30 HDs were collected into heparinized tubes. Then, peripheral blood lymphocytes (PBLs) was acquired, using human PBL isolation kit (P8900, Solarbio, China), and immediately used for the next step.
CD4+ T cells isolation and treatment
CD4+ T cells were freshly isolated from PBLs by via a negative selection principle using MagCellectTM human CD4+ T cell isolation kit (MAGH102, R&D Systems, USA) according to the provided instructions. The purity of CD4+ was above 92%. Freshly isolated CD4+ T were cultured in a complete RPMI media (Hyclone, USA) with 10% fetal calf serum (GIBCO, USA) and 100 U/mL penicillin (Solarbio) being added.
To assess the roles of CXCL4 in regulating Tregs percentage, freshly purified CD4+ T cells from patients were co-cultured with 200 and 500 µg/L of anti-CXCL4 antibody (Abcam Cat# ab9561, RRID:AB_308720) for 24 h. To study the involvement of STAT5 pathway in the promoted effect of CXCL4 protein on Tregs content, CD4+ T cells from healthy people were treated with 100 ng/mL of recombinant CXCL4 protein (795-P4-025, R&D), or 100 ng/mL of recombinant CXCL4 protein plus 10 µM of STAT5 inhibitor (Fox Chase Chemical Diversity, USA) (23).
Flow cytometric analysis of Tregs
CD4+ T cells were diluted with phosphate-buffered saline (106 cells/mL), and then stained with FITC-conjugated anti-human CD4 antibody (Thermo Fisher Scientific Cat# 11-0048-42, RRID:AB_1633390), APC-conjugated anti-human CD25 antibody (Thermo Fisher Scientific Cat# 17-0257-42, RRID:AB_11218671), or appropriate isotype control Abs (Mouse IgG1, kappa) for 30 min at 4 °C in darkness. For FOXP3 intracellular staining, after cell surface staining, cells were fixed by 2% formaldehyde and permeabilized using human FOXP3 Fix buffer (eBioscience) for 30 min, and then stained with PE-conjugated anti-human FOXP3 antibody (Thermo Fisher Scientific Cat# 12-4777-42, RRID:AB_1944444) or respective isotype control for 30 min. Tregs was defined using flow cytometry (BD Biosciences, San Jose, CA, USA). Live cell population of lymphocyte gated on the basis of FSC/SSC (gate P1) and P2 gating for CD4+ T cells positive cell population was set. The percentage of Tregs in CD4+ cells was calculated using FlowJo 7.6.1 software (Tree Star Inc., Ashland, OR, USA).
Western blot
After fully lysed, total protein within CD4+ T cells was quantified using BCA protein assay kit (Thermo), and 25 µg of which were separated using 15% SDS-PAGE. Proteins of CXCL4, chemokine receptor 3 (CXCR3), FOXP3, cytotoxic T lymphocyte antigen-4 (CTLA-4), STAT5 and p-STAT5 in the electrophoretic pure were transferred onto PVDF membranes (Millipore, USA) and incubated with the primary antibodies: antibody against CXCL4 (Abcam Cat# ab49735, RRID:AB_870744), CXCR3 (Ab154845, Abcam), FOXP3 (Abcam Cat# ab20034, RRID:AB_445284), CTLA-4 (Ab134090, Abcam), STAT5 (Abcam Cat# ab16276, RRID:AB_302363) and phosphorylated (p)-STAT5 (Abcam Cat# ab32364, RRID:AB_778105) and antibody against glyceraldehyde 3-phosphate dehydrogenase (Cell Signaling Technology Cat# 5174, RRID:AB_10622025) at 4oC overnight followed by secondary antibodies (Beyotime, Shanghai, China) for 1 hour at 25 °C. ECL system (GE Healthcare/Amersham Biosciences) was used for analysis. p-STAT5 was normalized using STAT5 while CXCL4, CXCR3, FOXP3, CTLA-4 and STAT5 were normalized using GAPDH.
Statistical analysis
Data was calculated from three independent experiment, analyzed using GraphPad Prism 7.0 software (La Jolla, CA, USA) and described as mean ± SEM (for serum sample analysis of CXCL4) or mean ± SD (for cell experiments). T-test (unpaired, two-tailed) was conducted for comparison between two groups and one-way ANOVA followed by Newman-Keuls post hoc tests was used for more than two groups. P values <0.05 were considered significant.
Results
CXCL4 was up-regulated in chronic osteomyelitis
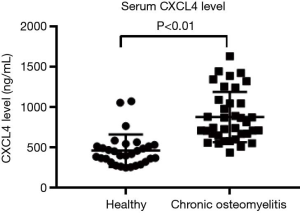
To study the roles of CXCL4 in chronic osteomyelitis, concentration of CXCL4 in serum samples of patients with chronic osteomyelitis (n=37) and healthy people (n=30) was assessed, using ELISA assay. As shown in Figure 1, CXCL4 was significantly enhanced when compared with corresponding healthy control, suggesting the involvement of CXCL4 in chronic osteomyelitis.
Anti-CXCL4 antibody inhibited Tregs percentage and suppressed the activation of STAT5 pathway in CD4+ T cells of patients with chronic osteomyelitis
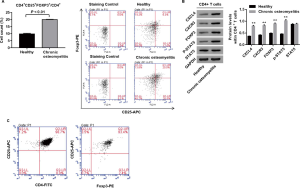
Figure S1 showed that Tregs percentage, and protein expression of CXCL4, CXCR3, FOXP3 and p-STAT5 was significantly enhanced within CD4+ T cells isolated from patients with chronic osteomyelitis when compared with the healthy control. To study the roles of CXCL4 in regulating Tregs content and the underlying mechanism, isolated CD4+ T cells (Figure S2) from patients with chronic osteomyelitis were exposure to IgG control and anti-CXCL4 antibody (200 and 500 µg/L), respectively. As shown in Figure 2A,B, anti-CXCL4 antibody decreased the percentage of Tregs in CD4+ T cells. Figure 2C,D showed a significant reduction in CXCL4 expression in CD4+ T cells after exposure to anti-CXCL4 antibody (200 and 500 µg/L), demonstrating a successful CXCL4 neutralization using monoclonal antibodies against CXCL4. Besides, anti-CXCL4 antibody decreased protein levels of CXCL4, CXCR3, FOXP3, CTLA-4 and p-STAT5 in a dose-dependent manner (Figure 2C,D), suggesting the inhibitory effect of anti-CXCL4 antibody on Tregs content in CD4+ T cells of patients, which was associated with the inactivation of STAT5 pathway.
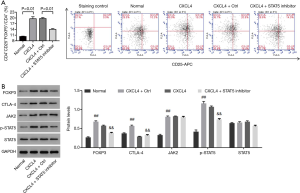
Recombinant CXCL4 protein promoted the percentage of Tregs in CD4+ T cells from healthy people via activating STAT5 pathway
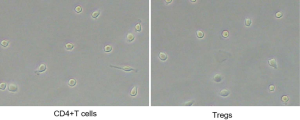
To study whether STAT5 pathway was the mechanism, by which CXCL4 regulated Tregs content in CD4+ T cells, CD4+ T cells isolated from healthy people were treated with recombinant CXCL4 (100 ng/mL), or recombinant CXCL4 (100 ng/mL) plus STAT5 inhibitor (10 µM), and then the percentage of Tregs, as well as protein levels of FOXP3, CTLA-4 and p-STAT5 within CD4+ T cells were assessed. As shown in Figure 3, with CXCL4 treatment, the proportion of Tregs as well as protein levels of FOXP3, CTLA-4 and p-STAT5 were significantly enhanced when compared with the normal cells. However, recombinant CXCL4-induced the changes of events mentioned above were significantly reversed with additional STAT5 inhibitor treatment, strengthening that recombinant CXCL4 protein increased Tregs percentage and function associated proteins via the activation of STAT5 pathway.
Discussion
Tregs were enhanced in chronic osteomyelitis (11). Active immunosuppression by Tregs induces peripheral tolerance to both self and foreign antigens in vivo. Thus, down-regulation of Tregs is a prospect potential to limit immunosuppression during chronic osteomyelitis. Chemokine CXCL10 and its receptor CXCR3 have been demonstrated promoted effect on the accumulation of Tregs (14). Herein, we found serum concentration of chemokine CXCL4 was up-regulated in chronic osteomyelitis (Figure 1). To study the effect of CXCL4 on Tregs content of chronic osteomyelitis, CD4+ T cells were isolated from chronic osteomyelitis patients. The in vitro data suggested that the percentage of Tregs/CD4+ T cells was remarkably inhibited in the presence of anti-CXCL4 antibody (Figure 2A), indicating that neutralization of CXCL4 attenuated the immunosuppression during chronic osteomyelitis by decreasing Tregs number. Conversely, recombinant CXCL4 significantly promoted the percentage of Tregs/CD4+ T cells of healthy people (Figure 3A), demonstrating that exogenous increase of CXCL4 contributed to enhanced Tregs numbers.
FOXP3 confers the regulatory activity of Tregs which not only regulates suppressive phenotype of Tregs, but also stabilizes the Tregs lineage (7,24). Loss of FOXP3 impairs immune suppressive activity of Tregs (25,26). Furthermore, Tregs constitutively express CTLA-4, the other marker of Tregs activation, which is not Treg-cell-specific or participates in in vitro immunosuppression (8). To elucidate the regulation of CXCL4 on Tregs at molecular levels, protein expression of FOXP3 and CTLA-4 within CD4+ T cells was assessed in the presence of anti-CXCL4 antibody or recombinant CXCL4 protein. Our results suggested that anti-CXCL4 antibody (200 and 500 µg/L) dose-dependently decreased FOXP3 and CTLA-4 (Figure 2C,D). Conversely, recombinant CXCL4 significantly increased FOXP3 and CTLA-4 (Figure 3B), indicating the promoted effect of CXCL4 on the activation and function of Tregs at a molecule level. Furthermore, evidence suggested that chemokine receptor CXCR3, secreted on circulating human Tregs, mediates the transportation of Tregs into the peripheral sites of inflammation (27). Inspiringly, our data firstly suggested the reduction of CXCR3 within Tregs in the presence of anti-CXCL4 antibody (Figure 2C,D).
Activation of STAT5 is sufficient to increase the numbers Tregs, favors FOXP3 expression in Tregs, and facilitates Treg cell function in vitro (28,29). STAT5 is a transcription factor, which is involved in cytosolic signaling and in mediating the expression of specific genes. Upon activation of JAKs through cytokine and growth factors stimulation, receptor associated and unphosphorylated STATs will be phosphorylated, which subsequently results in parallel dimerization and translocation to the nucleus to activate gene transcription (30). STAT5 pathway modulates Tregs function in asthmatics and diabetes (31,32). However, fewer reports have demonstrated the roles of STAT5 in regulating Tregs in chronic osteomyelitis. Our results indicated that the activation of STAT5 within CD4+ T cells was suppressed by anti-CXCL4 antibody while accelerated under recombinant CXCL4 treatment (Figures 2,3). Morcinek et al. (33) demonstrated that STAT5 could regulate the proliferation and apoptosis of cells, which is probably the cause of number changes in Tregs cell. Furthermore, recombinant CXCL4 induced the promoted effect on Tregs percentage and on the expression of FOXP3 and CTLA-4 within CD4+ T cells were significantly reversed by additional treatment of STAT5 inhibitor, substantiating that activation of STAT5 pathway was the mechanism, by which CXCL4 regulated Tregs content and activation in chronic osteomyelitis.
Conclusions
Anti-CXCL4 antibody inhibited Tregs percentage and reduced the expression of FOXP3 and CTLA-4 in CD4+ T cells, and blocking STAT5 activation was the underlying mechanism. Our data suggested that stimulation of CD4+ T cells with anti-CXCL4 antibody influenced Tregs content and its function. Further effort is needed to explore whether targeting CXCL4 is an immunotherapeutic strategy to block human chronic osteomyelitis.
Acknowledgments
Funding: The study was supported by the National Natural Science Foundation of China (No. 81603644), Natural Science Foundation of Zhejiang Province (LY20H270002), Zhejiang TCM Science and Technology Program (2020ZQ006), and Zhejiang Medical and Health Science and Technology Program (2020RC048).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-166
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-166). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province (No. 2016-016), and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent from the participants was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beck-Broichsitter BE, Smeets R, Heiland M. Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis 2015;28:240-5. [Crossref] [PubMed]
- Walter G, Kemmerer M, Kappler C, et al. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int 2012;109:257-64. [PubMed]
- Wang Y, Wang J, Meng J, et al. Epigenetic modification mediates the increase of LAG-3(+) T cells in chronic osteomyelitis. Inflammation 2017;40:414-21. [Crossref] [PubMed]
- Brady RA, Leid JG, Calhoun JH, et al. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 2008;52:13-22. [Crossref] [PubMed]
- Karpas K, Capousek M, Charvat J. Use of immunoactive treatment in the therapy of chronic osteomyelitis. Acta Chir Orthop Traumatol Cech 1983;50:378-82. [PubMed]
- Lieske N, Tonby K, Kvale D, et al. Targeting regulatory T cells in chronic inflammatory disease. (MPF2P.749). J Immunol 2015;194:63.8.
- Zhao H, Liao X, Kang Y. Tregs: where we are and what comes next? Front Immunol 2017;8:1578. [Crossref] [PubMed]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005;6:345-52. [Crossref] [PubMed]
- Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell 2008;133:775-87. [Crossref] [PubMed]
- Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol 2016;34:609-33. [Crossref] [PubMed]
- Wu Y, Tang Y, Liang X, et al. The role of increased frequency of treg cells in patients with chronic osteomyelitis. Orthopedics 2011;34:98. [PubMed]
- Himmel ME, Crome SQ, Ivison S, et al. Human CD4+ FOXP3+ regulatory T cells produce CXCL8 and recruit neutrophils. Eur J Immunol 2011;41:306-12. [Crossref] [PubMed]
- Altara R, Mallat Z, Booz GW, et al. The CXCL10/CXCR3 axis and cardiac inflammation: implications for immunotherapy to treat infectious and noninfectious diseases of the heart. J Immunol Res 2016;2016:4396368. [Crossref] [PubMed]
- Heller EA, Liu E, Tager AM, et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 2006;113:2301-12. [Crossref] [PubMed]
- Lee HT, Liu SP, Lin CH, et al. A Crucial Role of CXCL14 for promoting regulatory T cells activation in stroke. Theranostics 2017;7:855-75. [Crossref] [PubMed]
- von Hundelshausen P, Agten SM, Eckardt V, et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci Transl Med 2017;9:eaah6650.
- Fox JM, Kausar F, Day A, et al. CXCL4/Platelet Factor 4 is an agonist of CCR1 and drives human monocyte migration. Sci Rep 2018;8:9466. [Crossref] [PubMed]
- Nguyen TH, Medvedev N, Delcea M, et al. Anti-platelet factor 4/polyanion antibodies mediate a new mechanism of autoimmunity. Nat Commun 2017;8:14945. [Crossref] [PubMed]
- Affandi AJ, Silva-Cardoso SC, Garcia S, et al. CXCL4 is a novel inducer of human Th17 cells and correlates with IL-17 and IL-22 in psoriatic arthritis. Eur J Immunol 2018;48:522-31. [Crossref] [PubMed]
- Rani A, Murphy JJ. STAT5 in cancer and immunity. J Interferon Cytokine Res 2016;36:226-37. [Crossref] [PubMed]
- Van Raemdonck K, Gouwy M, Lepers SA, et al. CXCL4L1 and CXCL4 signaling in human lymphatic and microvascular endothelial cells and activated lymphocytes: involvement of mitogen-activated protein (MAP) kinases, Src and p70S6 kinase. Angiogenesis 2014;17:631-40. [Crossref] [PubMed]
- Mowafi F, Cagigi A, Matskova L, et al. Chemokine CXCL12 enhances proliferation in pre-B-ALL via STAT5 activation. Pediatr Blood Cancer 2008;50:812-7. [Crossref] [PubMed]
- Liao Z, Gu L, Vergalli J, et al. Structure-based screen identifies a potent small molecule inhibitor of Stat5a/b with therapeutic potential for prostate cancer and chronic myeloid leukemia. Mol Cancer Ther 2015;14:1777-93. [Crossref] [PubMed]
- Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature 2007;445:771-5. [Crossref] [PubMed]
- Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001;27:20-1. [Crossref] [PubMed]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330-6. [Crossref] [PubMed]
- Hoerning A, Koss K, Datta D, et al. Subsets of human CD4(+) regulatory T cells express the peripheral homing receptor CXCR3. Eur J Immunol 2011;41:2291-302. [Crossref] [PubMed]
- Cohen AC, Nadeau KC, Tu W, et al. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol 2006;177:2770-4. [Crossref] [PubMed]
- Antov A, Yang L, Vig M, et al. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J Immunol 2003;171:3435-41. [Crossref] [PubMed]
- Jiang L, Zhao XH, Mao YL, et al. Long non-coding RNA RP11-468E2.5 curtails colorectal cancer cell proliferation and stimulates apoptosis via the JAK/STAT signaling pathway by targeting STAT5 and STAT6. J Exp Clin Cancer Res 2019;38:465. [Crossref] [PubMed]
- Fohner A, Nguyen K, Krensky AM, et al. Lymphotactin improves treg function in asthmatics via the STAT5 pathway. Clin Immunol 2007;123:S77. [Crossref]
- Murawski MR, Litherland SA, Clare-Salzler MJ, et al. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Ann N Y Acad Sci 2006;1079:198-204. [Crossref] [PubMed]
- Morcinek JC, Weisser C, Geissinger E, et al. Activation of STAT5 triggers proliferation and contributes to anti-apoptotic signalling mediated by the oncogenic Xmrk kinase. Oncogene 2002;21:1668-78. [Crossref] [PubMed]


