An analysis of 20 clinical cases of refractory mycoplasma pneumonia in children
Introduction
Mycoplasma pneumoniae (MP) is one of the important pathogens of community-acquired pneumonia (CAP) in children. Mycoplasma pneumoniae pneumonia (MPP) accounts for 10% to 40% (1-6) of CAP in hospitalized children, which is a clinical concern of all pediatricians. Among them, refractory mycoplasma pneumonia (RMPP) cases have gradually increased in recent years (7). RMPP is more common in children than in adults, and its incidence is rising, with a rate of 51.6% in 2011, and has reached 84.6% in 2015 (7). Such high rate is associated with the excessive use of macrolides that cases resistance to this kind of antibiotics (7).
The pathogenesis of lung injury caused by RMPP is reported to be the amplified host immune response (8). For example, the excessive production of cytokines and overactivation of T-cell will cause various damages to the lung tissues (8). The infection as well as the lung tissue injuries will cause the typical clinical manifestations, including a persistent or repeated high fever, severe cough, and intrapulmonary and extrapulmonary complications (9). The condition is critical and, therefore, treatment is difficult. Commonly used treatments are antibiotics such as tetracyclines, fluoroquinolones, as well as corticosteroid and intravenous immunoglobin (9). However, due to the drug-resistance issue as talked above, treatment effects are often not very satisfactory (9).
The clinical data of 20 patients with RMPP admitted to the Pediatrics Department of the First Affiliated Hospital of Guangzhou Medical University in the recent 3 years were retrospectively analyzed. The clinical data of 36 patients with common mycoplasma pneumonia in the same period were compared. The clinical characteristics of RMPP were discussed in order to provide a reference for the diagnosis and treatment of RMPP.
We present the following article in accordance with the STROBE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-19-497).
Methods
General data
From January 2016 to December 2018, 86 children with MP pneumonia were selected from the Department of Pediatrics at the First Affiliated Hospital of Guangzhou Medical University. The refractory group included 20 cases of RMPP, 9 males (45%) and 11 females (55%); 1 case (5%) was younger than 3 years old, 5 cases (25%) were 3–5 years old, and 14 cases (70%) were 6–14 years old. In the general group, there were 36 cases, 16 males (44.44%) and 20 females (55.56%). There were 3 cases (8.33%) younger than 3 years old, 18 cases (50%) between 3 and 5 years old, and 15 cases (41.67%) between 6 and 14 years old. The family members of the selected children signed the informed consent and we obtained the approval of the hospital ethics committee. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2019K-50). This study was conducted in accordance with the declaration of Helsinki (as revised in 2013).
Entry criteria
All cases met the diagnostic criteria of MPP (8). The diagnosis of RMPP meets the following criteria: MPP can be diagnosed as RMPP (9) if the clinical symptoms of MPP are aggravated, the fever is still persistent, and pulmonary imaging is aggravated after 7 days or more after the regular treatment with macrolide antibiotics.
Exclusion of basic diseases
Congenital heart disease; bronchopulmonary dysplasia; Congenital Bronchopulmonary malformations (such as esophago-tracheal fistula, hernia, isolated lung, and tracheobronchial moderate-severe softening); hematological neoplasms; long-term use of hormones or immune agents; congenital immunodeficiency disease. Cases with incomplete data were also excluded.
Methods
The serum Mycoplasma pneumoniae antibody was detected by the gelatin particle agglutination test (PA) and the blood routine, C-reactive protein (CRP), procalcitonin (PCT), erythrocyte sedimentation rate (ESR), liver function, myocardial enzymes, and coagulation function were also detected. All patients underwent a chest X-ray examination and part of the patients underwent a chest CT examination.
Statistical methods
Data were analyzed using SPSS 15.0 statistical software. The measurement data of the two groups were compared by a t-test and the rate was compared by chi-square test. The difference was statistically significant (P<0.05).
Results
Comparison of the clinical characteristics between the two groups
There was no significant difference in the age and sex between the two groups (P>0.05); there were significant differences in heat duration, hospitalization time, hypoxemia, tachycardia, and lung rale between the two groups (P<0.05) but there was no significant difference in the symptoms of wheezing between the two groups (P>0.05), as shown in Table 1.

Full table
Comparison of the laboratory examination results between the two groups
There were significant differences in the peripheral blood leukocyte count, platelet count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and D dimer between the two groups (P<0.05), as shown in Table 2.

Full table
Comparison of the imaging data between the two groups
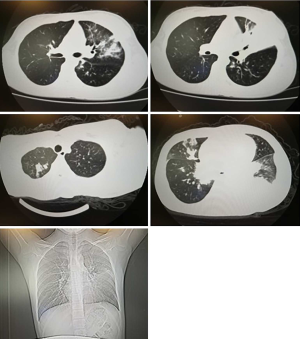
In the refractory group, 20 cases had large unilateral shadows and intrapulmonary complications. All cases had atelectasis. In the normal group, 8 cases had unilateral shadow and 10 cases had pulmonary complications, manifested as a small amount of unilateral pleural effusion, and enlarged hilar lymph nodes and atelectasis. There was a significant difference between the two groups (P<0.01), as shown in Table 3 and Figure 1.

Full table
Comparison of the extrapulmonary complications between the two groups
Extrapulmonary complications were found in the refractory group and 8 in the general group. There was a statistical difference between the two groups (P<0.01), as shown in Table 4.

Full table
Comparison of the treatment methods and prognosis between the two groups
Both groups were given standard treatment of macrolide antibiotics (azithromycin). There was a significant difference between the two groups in the azithromycin use time, hormones, C globules, liver-protecting drugs, heart-protecting drugs, and fiberoptic bronchoscopy (P<0.05), as shown in Table 5.

Full table
Discussion
The incidence of RMPP is increasing yearly. As such, how to diagnose and treat RMPP correctly has become a difficult problem for pediatricians (10-15). The main clinical features of RMPP were persistent high fever, coughing, a marked increase in inflammatory indicators (including CRP, LDH, SF), large consolidation on imaging, necrosis in some cases, and mucus embolism and/or mucosal necrosis under a bronchoscopy (16,17).
The results of this study showed that the duration of fever and hospitalization days in the RMPP group were significantly longer than those in the normal group and 45% of the cases had hypoxemia. Compared with the normal group, the performance of tachycardia and lung rale was prominent. Both groups had wheezing but there was no significant difference between the two groups. It is suggested that a long duration of fever, tachycardia, and lung rale protrusion may be the clinical characteristics of RMPP.
Compared with the normal group, CRP, ESR, and PCT in the RMPP group increased significantly and ALT and LDH in the liver function and cardiac enzymes increased significantly. White blood cell in the peripheral blood showed a trend of polarization, some cases showed a decrease, some cases showed an increase and platelet count and D dimer increased significantly. It is suggested that the increase of inflammatory markers should consider not only the mixed bacterial infection but also the immune damage caused by the MP infection. Wang et al. (18) reported that children with RMPP had significantly increased blood inflammatory markers, liver function damage, and cardiac enzymes. The results of this study correspond with these findings.
Chest X-ray films of RMPP may show one or more lobes with high-density consolidation, atelectasis or the extensive interstitial infiltration of both lungs often accompanied by moderate pleural effusion. At a later stage in the disease, the sub-segmental bronchi are partially or completely occluded, resulting in the consolidation and atelectasis of the lungs, which is difficult to improve and may even cause lung necrosis. It is easy to leave behind obstructive bronchiolitis and localized bronchiectasis. In this study, 20 cases of RMPP showed unilateral large shadow and pulmonary complications. All cases showed atelectasis and some cases showed hilar lymph node enlargement and pleural effusion. This study shows that the patients with unilateral pulmonary shadow and atelectasis need to be paid more attention as it is possible for these patients to develop RMPP.
The glycerophospholipids on the MP membrane share the same antigen composition with host cells. After infection, they can produce corresponding autoantibodies and induce an autoimmune response, which can cause extrapulmonary multiple system damage. RMPP can involve the nervous system, cardiovascular system, gastrointestinal tract, kidney, blood system, bone and joints, muscles, and skin, etc. The rash is easily confused with a drug-related rash and needs to be differentiated and diagnosed. However, neurological damage is relatively serious, and encephalitis is common whereas cardiac damage is mainly caused by elevated myocardial enzymes, arrhythmia, myocarditis, pericarditis, and sometimes heart failure. Hemolytic anemia is common in the blood system. In recent years, MP-related hemophagocytic lymph histiocytosis has been reported. In this study, we found that extrapulmonary complications occurred in the refractory group and 8 cases in the general group. There was a statistical difference between the two groups. From the systematic analysis of the involvement, the digestive system involvement is the most easily combined, which is the same as that of Jin et al.’s study (19).
When MPP children have atelectasis, pulmonary interstitial fibrosis, bronchiectasis or extrapulmonary complications, it has been generally agreed that glucocorticoids can be used. Soluble intercellular adhesion molecule 1 (sICAM-1) in the serum of infants with MP infection at the initial and convalescent stages increased significantly and glucocorticoid drugs could inhibit the expression of sICAM-1, thereby blocking the relevant immunological pathogenesis. The optimal timing of hormone use is uncertain. The dosage and course of treatment of hormones should be based on the clinical manifestations, CRP, and other inflammatory indicators shown through pulmonary imaging findings and multiple organ dysfunctions. This study showed that all patients in the RMPP group were treated with glucocorticoid, but the specific timing, route, and dosage of the drug are still worth exploring.
The treatment of RMPP needs to be standardized and macrolide antibiotics are used in foot therapy. Azithromycin has a hormone-like anti-inflammatory effect. There is sufficient evidence to confirm this effect in patients with cystic fibrosis and diffuse pan bronchiolitis. In the RMPP group, azithromycin was intravenously dripped for 2 courses, followed by oral sequential treatment. IVIG treatment is also used in MP extrapulmonary complications, such as neurological complications: encephalopathy or encephalitis, hemolytic anemia, etc. Three cases of RMPP were treated with gamma globulin for neurological and hematological complications. Compared with the normal group, the myocardial damage and liver function damage in the RMPP group were evident and the difference between the two groups was statistically significant. It was necessary to use liver-protecting and heart-protecting drugs for symptomatic treatment.
Fiberoptic bronchoscopy showed congestion and edema of the bronchial mucosa at the lesion site, adherence of mucous secretions, partial bronchial insufficiency, tuberous protuberances of the wall mucosa, and a narrow opening of the lumen. For large consolidation and atelectasis cases, fiberoptic bronchoscopy can go deep into the local area, wash, clean mucus plugs, produce secretion drainage, and reduce obstructions. In this study, 20 cases of RMPP were examined and treated by fiberoptic bronchoscopy.
Conclusions
A long duration of fever, tachycardia, and lung rale protrusion may be the clinical characteristics of RMPP. Unilateral pulmonary shadow and atelectasis should be paid more attention, which may be a high-risk factor for the development of RMPP. The inflammation index of RMPP cases has increased and there were many complications inside and outside the patient’s lungs. It was necessary to give enough macrolides to fight the infection and use Glucocorticoid and Intravenous immunoglobulin reasonably, while liver, heart, and fiberoptic bronchoscopy were completed to improve the effectiveness of diagnosis and treatment.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding: This study was funded by the Guangzhou Science and Technology Innovation Project (201607010373) and Project supported by National Natural Science Foundation of China (81770063). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnote
Reporting Checklist: The authors have completed the STROBE Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-19-497
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-497
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-497). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted with approval from the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2019K-50). This study was conducted in accordance with the declaration of Helsinki (as revised in 2013). Parents of all patients signed a document of informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leung AKC, Wong AHC, Hon KL. Community-Acquired Pneumonia in Children. Recent Pat Inflamm Allergy Drug Discov 2018;12:136-44. [Crossref] [PubMed]
- Belon AP, Serrano-Lomelin J, Nykiforuk CIJ, et al. Health gradients in emergency visits and hospitalisations for paediatric respiratory diseases: A population-based retrospective cohort study. Paediatr Perinat Epidemiol 2020;34:150-60. [Crossref] [PubMed]
- Gao LW, Yin J, Hu YH, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect 2019;147:e192. [Crossref] [PubMed]
- Dai W, Wang H, Zhou Q, et al. An integrated respiratory microbial gene catalogue to better understand the microbial aetiology of Mycoplasma pneumoniae pneumonia. Gigascience 2019;8:giz093. [Crossref] [PubMed]
- Kurkela S, Puolakkainen M, Hokynar K, et al. Mycoplasma pneumoniae outbreak, Southeastern Finland, 2017-2018: molecular epidemiology and laboratory diagnostic lessons. Eur J Clin Microbiol Infect Dis 2019. [Crossref] [PubMed]
- Lee YH, Seo H, Cha SI, et al. A case of pseudomembranous tracheitis caused by Mycoplasma pneumoniae in an immunocompetent patient. Ann Transl Med 2019;7:205. [PubMed]
- Søndergaard MJ, Friis MB, Hansen DS, et al. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. PLoS One 2018;13:e0195288. [PubMed]
- Rogozinski LE, Alverson BK, Biondi EA. Diagnosis and treatment of Mycoplasma pneumoniae in children. Minerva Pediatr 2017;69:156-60. [PubMed]
- Wang Y, Ma L, Li Y, et al. Epidemiology and clinical characteristics of pathogens positive in hospitalized children with segmental/lobar pattern pneumonia. BMC Infect Dis 2020;20:205. [Crossref] [PubMed]
- Xu JJ, Shu LH. Clinical characteristics of refractory Mycoplasma pneumoniae pneumonia in children. Zhongguo Dang Dai Er Ke Za Zhi 2018;20:37-42. [PubMed]
- Huang L, Chen H, Peng S. Spontaneous pneumomediastinum, emphysema, and pulmonary bullae associated with refractory Mycoplasma pneumoniae pneumonia in a child. Pediatr Pulmonol 2017;52:E77-80. [Crossref] [PubMed]
- Yang HJ, Song DJ, Shim JY. Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr 2017;60:167-74. [Crossref] [PubMed]
- Han R, Yu Q, Zhang G, et al. Comparison of azithromycin and erythromycin in the treatment of mycoplasma pneumonia in children. Pak J Med Sci 2020;36:156-9. [PubMed]
- Ranjbar R, Halaji M. Epidemiology of Mycoplasma pneumoniae prevalence in Iranian patients: a systematic review and meta-analysis. J Med Microbiol 2019;68:1614-21. [PubMed]
- Cheng S, Lin J, Zheng X, et al. Development and validation of a simple-to-use nomogram for predicting refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 2020;55:968-74. [Crossref] [PubMed]
- Liu J, Zhao F, Lu J, et al. High Mycoplasma pneumoniae loads and persistent long-term Mycoplasma pneumoniae DNA in lower airway associated with severity of pediatric Mycoplasma pneumoniae pneumonia. BMC Infect Dis 2019;19:1045. [Crossref] [PubMed]
- Fan H, Lu B, Yang D, et al. Distribution and Expression of IL-17 and Related Cytokines in Children with Mycoplasma pneumoniae Pneumonia. Jpn J Infect Dis 2019;72:387-93. [Crossref] [PubMed]
- Wang Y, Zhang Y, Lu W, et al. Serum Tumor Necrosis Factor-α and Interferon-γ Levels in Pediatric Mycoplasma pneumoniae Pneumonia: A Systematic Review and Meta-Analysis. Can Respir J 2018;2018:8354892. [Crossref] [PubMed]
- Jin X, Zou Y, Zhai J, et al. Refractory Mycoplasma pneumoniae pneumonia with concomitant acute cerebral infarction in a child: A case report and literature review. Medicine (Baltimore) 2018;97:e0103. [Crossref] [PubMed]