
The correlation between plasma trimethylamine N-oxide level and heart failure classification in northern Chinese patients
Introduction
Cardiovascular disease represents the largest single contributor toward global mortality, and is poorly controlled in approximately 40% of patient deaths in China (1-3). Heart failure (HF) is the end phase of cardiovascular diseases and remains a public health issues in many countries, which is a main cause of cardiovascular mortality worldwide. At present, the diagnosis and prognostication of HF is most commonly determined by brain natriuretic peptide (BNP) and N-terminal probrain natriuretic peptide (NT-proBNP). Plasma NT-proBNP is an excellent predictive risk factor for HF. However, the level of NT-proBNP changes following therapy (4).
Trimethylamine N-oxide (TMAO), a metabolite from choline and L-carnitine, is produced by gut microbiota (5-7). At present, many studies have indicated a relation between plasma levels of TMAO and cardiovascular diseases, and found that TMAO was an independent risk factor for major adverse cardiac events (8-12). Our previous study revealed that plasma level of TMAO was a risk factor for coronary heart disease in Chinese patients (13). In the present study, we determined plasma levels of TMAO in patients with or without HF. The diagnostic ability of TMAO was also evaluated relative to the well-established NT-proBNP. The previous research had demonstrated that TMAO was not only a predictor of cardiovascular diseases, but also directly increased the risk of death from cardiovascular diseases. Therefore, TMAO may be a new strategy for diagnosis and treatment of HF.
This study will explore the correlation between plasma TMAO level and HF in northern China. The severity of HF will be assessed by determining the plasma TMAO level in patients with HF. To determine the diagnostic value of TMAO in HF, TMAO was also evaluated relative to the well-established biomarker NT-proBNP. We can obtain new ideas for clinical prevention and treatment of HF. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-296).
Methods
Study population
During the period of May 2016 to August 2017, 296 patients with or without HF were recruited through the Department of Cardiology, First Affiliated Hospital of the Harbin Medical University. The inclusion criteria for the HF group were chronic HF patients, diagnosed by a cardiologist. Patients were excluded from the study if they had malignant tumor, simultaneous infection or severe hepatic and renal dysfunction diseases. The control group consisted of 112 participants with no clinical history of HF. The HF patients further were assigned to 3 groups according to the functional classification system of the New York Heart Association (NYHA), which included the NYHA II (n=8), NYHA III (n=82), and NYHA IV (n=94) groups. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Committees of the First Affiliated Hospital of Harbin Medical University (No. IRB-AF/SC-08/05.0) and individual consent for this retrospective analysis was waived.
Laboratory testing
The recruited participants had the medical examinations carried out and were assessed by specialized cardiologist who gathered information about HF-related symptoms such as NYHA class. Serum levels of biochemical parameters were tested in the clinical laboratory of the First Affiliated Hospital of the Harbin Medical University. Echocardiography was conducted by investigators who were blinded to this study.
Quantification of plasma TMAO levels
The plasma was collected from each participant and then stored at –80 °C refrigerator until analysis. Plasma level of TMAO was determined by LC-MS/MS as described previously (14). Five µL of each sample supernatant was injected into a silica column (100 mm × 2 mm, 3 µm, Phenomenex Luna Silica, USA) and was determined by high performance liquid chromatography system (Agilent Technologies, USA). TMAO and d9-TMAO were monitored using a triple quadrupole mass spectrometer (AB Sciex, USA).
Data analysis and statistics
Continuous variables were demonstrated as a mean ± SD, if a normal distribution was detected. Differences between 2 independent groups were analyzed using an independent-samples t-test, while differences among multiple groups were assessed using a one-way ANOVA. Continuous variables were demonstrated as a median and interquartile range, if not normally distributed. Differences between 2 independent groups were analyzed using the Mann-Whitney U test, while differences among multiple independent groups were analyzed using the Kruskal-Wallis test. Categorical clinical variables were displayed as % and were compared using the chi-square test. The receiver operating characteristic (ROC) curves were drawn and the area under the curve (AUC) was calculated to assess the HF risk. Logistic regression analysis was applied to evaluate the association between the TMAO and HF risks. The correlation analysis for the association of TMAO with risk factors of HF was assessed by Pearson’s correlation analysis (for continuous data) or Spearman’s correlation analysis (for categorical data). All statistical assessments were performed using SPSS 20.0 software. Statistical significance for all analyses was accepted at P<0.05.
Results
Patient characteristics
This study consisted of 112 controls and 184 HF patients (Table 1). Patients with HF were classified as NYHA II (n=8), NYHA III (n=82) or NYHA IV (n=94) based on their functional and clinical status (Table 2). The differences of age, albumin, hemoglobin, ALT, TC and TG among the NYHA II, NYHA III and NYHA IV groups were not significantly different. The differences of LVEF, LAD, LVEDD, BUN, creatinine and NT-proBNP among the NYHA II, NYHA III and NYHA IV groups were significantly different (P<0.01).
Full table
Full table
Elevated plasma TMAO levels in HF patients
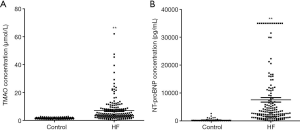
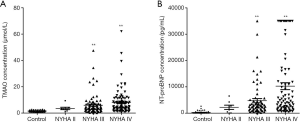
TMAO was isolated from plasma samples of control subjects and HF patients. The plasma levels of TMAO were analyzed using LC-MS/MS. TMAO was 7.0±0.6 µmol/L in HF group and 1.5±0.1 µmol/L in control group (P<0.01; Figure 1A). NT-proBNP were higher in HF patients than in controls (P<0.01; Figure 1B). These results indicated that TMAO or NT-proBNP have a significant association with HF (P<0.01). Additionally, TMAO and NT-proBNP levels were determined in the 3 HF groups relative to the control group (Figure 2A,B). The plasma levels of TMAO were 3.5±0.9, 6.0±0.8 and 8.1±1.0 µmol/L in NYHA II, NYHA III and NYHA IV groups, respectively. The plasma levels of NT-proBNP increased from NYHA II to NYHA IV group. As expected, both TMAO and NT-proBNP in all HF groups were significantly higher than in the control group.
Assessment of TMAO diagnostic potential
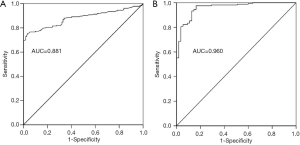
To investigate whether TMAO can discriminate between the HF and the control groups, ROC curves were constructed for TMAO and NT-proBNP. The AUC value for TMAO was 0.881 (95% CI: 0.842–0.920; P<0.01) and was 0.960 for NT-proBNP (95% CI: 0.934–0.986; P<0.01) (Figure 3A,B). The diagnostic ability of TMAO was lower than that of NT-proBNP. The univariate analysis with logistic regression indicated that the odds ratios (OR) value was 3.944 (95% CI: 2.702–5.757) and 1.003 (95% CI: 1.002–1.004) for TMAO and NT-proBNP between control and HF, respectively (Table 3). The multivariate logistic regression analysis showed: the OR values was 4.849 (95% CI: 1.885–12.474) and 1.002 (95% CI: 1.001–1.004) for TMAO and NT-proBNP between control and HF, respectively (Table 4).
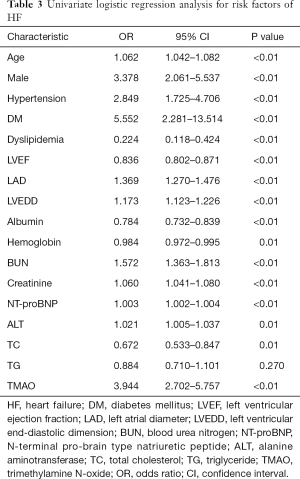
Full table
Full table
Correlation of TMAO with clinical parameters of HF
The correlation analysis was used to indicate correlations between TMAO and other risk factors for HF. The results indicated that TMAO was correlated with NT-proBNP, albumin, hemoglobin, BUN and creatinine (P<0.01; Table 5). These results showed that TMAO was significantly correlated with risk factors for HF.
Full table
Comparison of the TMAO diagnostic efficacy in HF classification
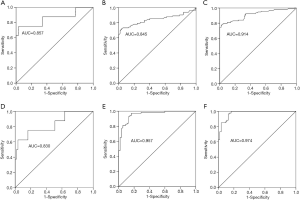
To investigate the diagnostic value of TMAO and NT-proBNP among the NYHA functional class II to IV HF, ROC curves of TMAO and NT-proBNP were examined in the 3 HF groups relative to the control group (Figure 4A,B,C,D,E,F). The results indicated that both TMAO and NT-proBNP depicted significant values for the AUC. The AUC value for TMAO was 0.857 (95% CI: 0.674–1.000; P<0.01), 0.845 (95% CI: 0.778–0.911; P<0.01) and 0.914 (95% CI: 0.872–0.956; P<0.01) in NYHA II, NYHA III and NYHA IV groups, respectively. The AUC value for NT-proBNP was 0.830 (95% CI: 0.657–1.000; P<0.01), 0.957 (95% CI: 0.924–0.989; P<0.01) and 0.974 (95% CI: 0.953–0.996; P<0.01) in NYHA II, NYHA III and NYHA IV groups, respectively.
Discussion
In this study, we determined the plasma levels of TMAO among patients with or without HF, and identified the correlation between TMAO and HF classification in northern Chinese patients. The plasma TMAO levels of the HF patients were significantly upregulated relative to the controls. The plasma levels of TMAO were increased from NYHA II to NYHA IV group. TMAO may serve as potential HF diagnostic markers for northern Chinese patients. As expected, the levels of NT-proBNP in NYHA II to NYHA IV HF groups were also significantly higher than in the control group. Thus, the well-established biomarker NT-proBNP was more effective than TMAO as a biomarker for detecting HF.
Many biomarkers have changed in HF, which reflect hemodynamic status, inflammation, and collagen homeostasis (15-17). At present, the mainly diagnostic index of HF is left ventricular ejection fraction (LVEF) and NT-proBNP. LVEF value is an important index for evaluating HF, however, which is not suitable for HF with a preserved EF. Meantime, the increase of NT-proBNP was not only in HF, but also in many other cardiovascular diseases (18,19). The plasma level of NT-proBNP also could change with the patient’s age, which might be related to the changes of cardiac microstructure (20). So, it is very important to find biomarkers for diagnosis of HF.
Accumulating data have demonstrated an association between TMAO and HF (9,10). These studies indicated that TMAO was a risk factor for HF, which indicated higher mortality risk in western countries (21). TMAO is produced from metabolism of nutrients by intestinal bacteria, because of the different dietary structure between western country and China, the relation between TMAO level and HF in northern Chinese patients is unclear. In this study, the plasma level of TMAO was 7.0±0.6 µmol/L in HF group and 1.5±0.1 µmol/L in control group (P<0.01; Figure 1A). Our results found that TMAO have a significant association with HF in northern Chinese patients. Furthermore, the ROC analysis revealed that AUC of TMAO was 0.881 to predict HF patients. Logistic regression analysis showed that TMAO was an independent predictor of HF. These findings suggest that TMAO may facilitate accurate diagnosis of HF and serve as an improved prognostic tool for northern Chinese patients. In this study, HF patients were further divided into NYHA II to NYHA IV groups. The plasma levels of TMAO were remarkably increased from NYHA II to NYHA IV group (3.5±0.9, 6.0±0.8 and 8.1±1.0 µmol/L, respectively). The ROC revealed that AUC of TMAO was 0.857 (95% CI: 0.674–1.000; P<0.01), 0.845 (95% CI: 0.778–0.911; P<0.01) and 0.914 (95% CI: 0.872–0.956; P<0.01) in NYHA II, NYHA III and NYHA IV groups, respectively. These results indicated that TMAO was an independent predictor of HF, and was highly associated with HF classification in northern Chinese patients. The exact role of TMAO on HF is unclear. Many studies have found that dietary intake affected gut microbiome and TMAO level had been associated with HF outcomes (22,23). High plasma level of TMAO was associated with diastolic dysfunction and mortality (10,21). In an experimental research, mice were fed with TMAO could induce HF, and increased ventricular remodelling (24). The previous study indicated that TMAO promoted proliferation, migration and collagen secretion via activating the NLRP3 inflammasome in cultured cardiac fibroblast (25). TMAO levels were significantly elevated in TAC-induced cardiac hypertrophy in SD rats. Furthermore, TMAO directly stimulated cardiac hypertrophy and fibrosis involving Smad3 signaling in vitro and in vivo (26). These results may be the possible mechanism of TMAO induced HF.
The present study had limitations. First, it had a relatively small study population. The number of NYHA II enrolled was very little. Second, our study was an observational and retrospective study, the further prospective data regarding the relation between TMAO and mortality of HF in patients are needed. Third, the exact role and mechanism of TMAO on HF still needs to be further studied.
Conclusions
TMAO was an independent predictor of HF and associated with HF classification in northern Chinese patients. The importance of our study is emphasized by the decrease of TMAO, offering new strategies for treatment of HF.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 81400250, 81870169), Natural Science Foundation of Heilongjiang Province (H2017040).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-296
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-296
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-296). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Committees of the First Affiliated Hospital of Harbin Medical University (No. IRB-AF/SC-08/05.0) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu S, Li Y, Zeng X, et al. Burden of Cardiovascular Diseases in China, 1990-2016 Findings From the 2016 Global Burden of Disease Study. JAMA Cardiol 2019;4:342-52. [Crossref] [PubMed]
- Sacco RL, Roth GA, Reddy KS, et al. The heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American Heart Association and World Heart Federation. Circulation 2016;133:e674-90. [Crossref] [PubMed]
- Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 2016;387:251-72. [Crossref] [PubMed]
- Lai MY, Kan WC, Huang YT, et al. The Predictivity of N-Terminal Pro b-Type Natriuretic Peptide for All-Cause Mortality in Various Follow-Up Periods among Heart Failure Patients. J Clin Med 2019;8:357. [Crossref] [PubMed]
- Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 2018;16:171-81. [Crossref] [PubMed]
- Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 2013;83:1010-6. [Crossref] [PubMed]
- Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine Noxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015;116:448-55. [Crossref] [PubMed]
- Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904-10. [Crossref] [PubMed]
- Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014;64:1908-14. [Crossref] [PubMed]
- Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail 2015;21:91-6. [Crossref] [PubMed]
- Senthong V, Li XS, Hudec T, et al. Plasma trimethylamine N-Oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol 2016;67:2620-8. [Crossref] [PubMed]
- Senthong V, Wang Z, Li XS, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc 2016;5:e002816. [Crossref] [PubMed]
- Dong Z, Liang Z, Guo M, et al. The association between plasma levels of Trimethylamine N-oxide and the risk of coronary heart disease in Chinese patients with or without type 2 diabetes mellitus. Dis Markers 2018;2018:1578320. [Crossref] [PubMed]
- Liang Z, Dong Z, Guo M, et al. Trimethylamine N-oxide as a risk marker for ischemic stroke in patients with atrial fibrillation. J Biochem Mol Toxicol 2019;33:e22246. [Crossref] [PubMed]
- Schwermer K, Hoppe K, Radziszewska D, et al. N-terminal pro-B-type natriuretic peptide as a marker of hypervolemia and predictor of increased mortality in patients on hemodialysis. Pol Arch Med Wewn 2015;125:560-9. [PubMed]
- Marra AM, Arcopinto M, Salzano A, et al. Detectable interleukin-9 plasma levels are associated with impaired cardiopulmonary functional capacity and all-cause mortality in patients with chronic heart failure. Int J Cardiol 2016;209:114-7. [Crossref] [PubMed]
- López B, Ravassa S, González A, et al. Myocardial Collagen Cross-Linking Is Associated With Heart Failure Hospitalization in Patients With Hypertensive Heart Failure. J Am Coll Cardiol 2016;67:251-60. [Crossref] [PubMed]
- Dudink EA, Weijs B, Tull S, et al. The Biomarkers NT-proBNP and CA-125 are Elevated in Patients with Idiopathic Atrial Fibrillation. J Atr Fibrillation 2018;11:2058. [Crossref] [PubMed]
- Madan N, Lee AK, Matsushita K, et al. Relation of Isolated Systolic Hypertension and Pulse Pressure to High-Sensitivity Cardiac Troponin-T and N-Terminal pro-B-Type Natriuretic Peptide in Older Adults (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2019;124:245-52. [Crossref] [PubMed]
- Gao P, Zhu Q, Bian S, et al. Prognostic value of plasma NT-proBNP levels in very old patients with moderate renal insufficiency in China. Z Gerontol Geriatr 2018;51:889-96. [Crossref] [PubMed]
- Trøseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015;277:717-26. [Crossref] [PubMed]
- Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013;62:485-95. [Crossref] [PubMed]
- Nagatomo Y, Tang WH. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail 2015;21:973-80. [Crossref] [PubMed]
- Organ CL, Otsuka H, Bhushan S, et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail 2016;9:e002314. [Crossref] [PubMed]
- Li X, Geng J, Zhao J, Ni Q, et al. Trimethylamine N-Oxide Exacerbates Cardiac Fibrosis via Activating the NLRP3 Inflammasome. Front Physiol 2019;10:866. [Crossref] [PubMed]
- Li Z, Wu Z, Yan J, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 2019;99:346-57. [Crossref] [PubMed]


