Diabetes and multidrug-resistance gene mutation: tuberculosis in Zunyi, Southwest China
Introduction
Tuberculosis (TB) and diabetes mellitus (DM) are both chronic diseases, and when they co-occur, they can be aggravated by mutual influence (1). DM has been shown to negatively impact TB treatment outcomes that the risk of TB disease is increased over 3-fold in people with diabetes compared to people without DM (2). TB can aggravate the disorder of glucose metabolism and make the blood sugar level of DM patients difficult to control. DM can lead to protein and fat metabolism disorders, which can reduce immune function and aggravate the condition of TB patients (3,4). DM has been shown to have a negative impact on immune function through the accumulation of advanced glycation end-products that alter phagocyte function (5). TB patients with DM is treated with multidrug therapy [rifampin (RIF) and isoniazid (INH)] for the 6 months of treatment compare with TB patients, TB treatment outcomes are worse in patients with DM (6). The risk of death or treatment failure was 1.7 times higher in TB patients with DM than in those without DM (7). However, an important problem in TB treatment is anti-mycobacterial resistance. DM patients with TB are also more likely to develop multidrug-resistant TB (MDR-TB) (8). 16% diabetic TB patients showed multi drug resistance (MDR) which was three times higher than resistance (5.9%) found in non-diabetic TB patients (9). RIF and INH is the two most effective anti-TB drugs, resistance to RIF and INH is multidrug-resistant (MDR) TB. Rifampin resistance is conferred by chromosomal mutations in the rpoB gene encoding the β subunit of the RNA polymerase (RpoB). Most rifampin-resistance-conferring mutations in Mtb clinical isolates are due to amino acid changes at codons 531, 526, 522, and 513 of RpoB (10). The mutation of katG or inhA is the major mechanism of INH resistance. KatG S315 mutation is the most common mutation in INH-resistant strains and accounts for more than half of the resistant clinical isolates (11). Furthermore, accompanying multidrug resistance will greatly increase the difficulty of treating the disease.
There were 10 million new cases of TB worldwide in 2018, with China accounting for 9% of cases and ranking second in the world in incidence (12). Guizhou province is a high incidence area and has the fourth highest incidence rate of TB in China, with the highest drug-resistance rate. Cases are mainly concentrated around Zunyi, Bijie, Tongren, and southeast Guizhou (13). In particular, the emergence of multidrug-resistant tuberculosis (MDR-TB) has brought more serious challenges to the prevention and control of TB worldwide (14). MDR-TB is a condition in which Mycobacterium tuberculosis is at least resistant to rifampin (RIF) and isoniazid (INH) (15). RIF and INH, as key first-line drugs in anti-TB treatment programs, have always played an important role in the treatment of TB. However, the emergence of MDR-TB may be related to the mutations of the rpoB, katG, and inhA genes of the two drug-related targets (16,17). This study selected TB patients in Zunyi and used polymerase chain reaction (PCR) to detect mycobacterial DNA and RNA (18) for the purpose of investigating the correlation between TB and diabetes mellitus with tuberculosis (DM-TB) in Zunyi. It is hoped the findings can clarify the relationship between the resistance-causing MTB-TB mutations of rpoB, katG, and inhA and the molecular biological characteristics of MDR-TB. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1368).
Methods
General information
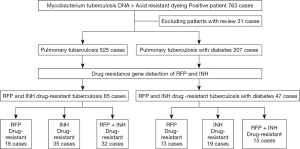
This study collected sputum or irrigating fluid specimens from 763 pulmonary TB patients (according to the WS 288-2017 diagnostic criteria for pulmonary TB, Table 1) treated in the Department of Respiratory and Intensive Medicine of our hospital from December 2017 to July 2019. We detected the DNA, RNA, and acid-fast staining of Mycobacterium tuberculosis and yielded positive results for rifampicin and isoniazid resistance gene testing (primer sequences for rifampin and isoniazid resistance detection, Table 2) . We then compared the correlation between multidrug resistance in TB patients and TB-DM patients (according to the American Diabetes Association 2013 standardization diagnosis and treatment guidelines for diabetes, Table 1). Meanwhile, we collected the relevant clinical data of the included patients. The research process is shown in Figure 1.

Full table

Full table
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Guizhou Aerospace Hospital Medical Ethics Management Committee, and written informed consent was obtained from all patients.
Main instruments and reagents
The main equipment used was Hongshi SLAN-96P fluorescence quantitative PCR instrument (Shanghai, China), ABI-2710 Nucleic acid Amplifier (Applied Biosystems, USA), Yaneng YN-H16 Constant Temperature Hybridization Instrument (Shenzhen, China), Rendu FZP-1 Nucleic Acid Purification Instrument (Shanghai, China), Aosheng Micro Spectrophotometer Nano-100 (Hangzhou, China). A Mycobacterium nucleic acid detection kit (PCR-fluorescent Probe Method; article number 301031) was obtained from Chengdu Boao Jingxin Biotechnology Co, Ltd (Chengdu, China). A Mycobacterium TB nucleic acid detection kit (RNA constant temperature amplification; article number 20153401875) was acquired from Shanghai Rendu Biological Technology Co, Ltd (Shanghai, China). The Mycobacterium TB rifampicin resistance gene detection kit (PCR-reverse Dot Hybridization Method; article number 20153400356) was provided by Asia Energy Biotechnology Co, Ltd (Shenzhen, China).
MTB DNA and RNA testing
MTB DNA and RNA extraction
An equal volume of sodium hydroxide was added (concentration of 4%) to the patient’s sputum or bronchial lavage fluid sample, shaken, mixed, and then left to stand for 15 minutes to fully liquefy. This mixture was centrifuged at 12,000 rpm/min for 10 minutes, and the supernatant was discarded afterwards. Next, 1 mL of cleaning solution (with the main component being normal saline) was added and mixed well; the mixture was centrifuged at 12,000 rpm/min for 5 minutes, and the supernatant was discarded afterwards. The pellet was divided into two tubes with 50 µL of nucleic acid extract (for DNA extraction) and 50 µL of dilution being added (for RNA extraction), with ultrasonic crack applied for 10 minutes. The DNA extraction tube was placed in a shaking metal bath. The tube was shaken at 1,800 rpm/min at 100 °C for 10 min, centrifuged at 12,000 rpm/min for 5 minutes, and the supernatant was collected. The RNA extraction tube was directly centrifuged at 12,000 rpm/min for 5 min, and the supernatant was collected.
MTB DNA and RNA testing
To begin, 20 µL of MTB DNA PCR reaction system was formed using 18 µL of probes, Taq enzymes, and primers, along with 2 µL of templates. The amplification procedure included 1 cycle at 37 °C for 300 s and at 94 °C for 180 s; 40 cycles at 94 °C for 15 s and at 60 °C for 30 s; and 1 cycle at 50 °C for 10 s. Then, 32µL of MTB RNA PCR reaction system was formed using 30 µL of probes, reverse transcriptases, and primers, along with 2 µL of templates. The amplification program was 40 cycles of constant temperature amplification at 42 °C for 60 s. The results were interpreted as positive, and the DNA amplification products were kept in the refrigerator at −20 °C.
Acid-fast staining
The experiment was completed by our laboratory. Briefly, 2–3 drops of carbohydrate acid red were used after initial dyeing, and the slide was rinsed with water, decolorized with 3% hydrochloric acid alcohol for 30 seconds to 1 minute, rinsed with water again, washed with alkaline Meilan solution for 1 minute, and washed with water. After the slide had absorbed the water, 1–2 drops of cedar oil on the glass slide, carefully observe and count under a high magnification lens (also called oil lens). The number of MTB DNA- and RNA-positive subjects was counted, and the positive acid-fast staining results were compared. Then, the relevant clinical information of the above patients was collected.
Blood glucose measurement
The blood glucose and fasting blood glucose of the included patients were tested. According to the diagnostic criteria in Table 1, the patients were divided into a simple tuberculosis group and a tuberculosis combined with diabetes group.
PCR-reverse dot hybridization
PCR amplification
First, 25 µL of reaction system were formed using 21 µL of dNTP, Taq enzyme, and primers, along with 4 µL of template DNA. The amplification program was at 50 °C for 120 s and at 94 °C for 600 s; at 95 °C for 45 s through 1 cycle, at 68 °C for 60 s through 30 cycles; at 95 °C for 30 s, at 54 °C for 30 s, and at 68 °C for 60 s through 30 cycles; and at 68 °C for 600 s through 1 cycle. Table 3 summarizes the mutation sites of rifampin- and isoniazid- resistance testing.

Full table
Hybridization and color development
The strips were numbered and placed in a 15 mL centrifuge tube containing PCR amplification product buffer. After 10 min at 100 °C and 90 min at 59 °C, the membranes strip was placed into a 50 mL centrifuge tube containing sodium citrate buffer, and washed gently at 59 °C for 15 min. The membranes were stripped into the coloring solution containing horseradish peroxidase for 10 min, the corresponding coloring point was observed, and the mutations of rpoB, katGm and inhA genes were interpreted.
Statistical analysis
SPSS 22. 0 statistical software package was used for analysis. The measurement data conforming to the normal distribution are expressed as
Results
Analysis of related factors
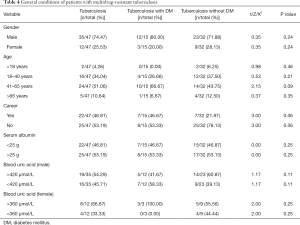
In the comparisons of gender, age, occupation, and other factors, along with the serum albumin test results of TB patients and TB-DM patients, the differences between the groups were found to not be statistically significant (Table 4). However, in the TB and diabetes group, gender (χ2=10.80, P<0.01), age (χ2=21.60, P<0.01) and female blood uric acid level (χ2=6.00, P<0.05), and other related factors showed statistical differences. In the simple TB group, gender (χ2=12.25, P<0.01), age (χ2=17.33, P<0.01), occupation (χ2=20.25, P<0.01) and other related factors showed statistical differences.
Full table
PCR-reverse dot hybridization test results
rpoB, katG, and inhA genes were detected in 732 patients with pulmonary TB, including 32 cases of multidrug resistance and 15 cases of multidrug resistance combined with diabetes (Figure 2).

Mutation rate of drug resistance sites in patients with MDR-TB
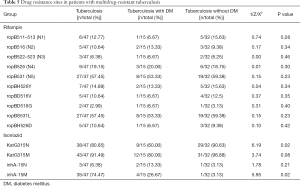
In the comparison of 32 patients with MDR and 15 patients with MDR combined with diabetes concerning the mutation rate of rpoB, katG, and inhA gene sites, patients with simple MDR-TB were found to be prone to KatG315N site mutation, while InhA-15M mutations were more likely to occur in patients with diabetes combined with MDR-TB. However, the differences between the remaining groups were not statistically significant (Table 5).
Full table
Distribution of resistance gene rpoB mutation sites
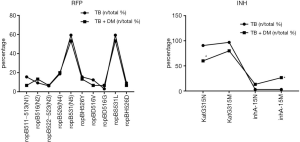
When rifampicin resistance occurred in patients with MDR-TB, mutations at ropB531 and ropBS531L sites were most likely to concomitantly occur. For isoniazid resistance, the mutation rate at the KatG gene was higher, and MDR-TB patients were prone to the KatG315N site mutation. Diabetes patients with MDR-TB were more prone to inhA-15M site mutation, but there were no obvious differences in other mutation sites (Figure 3).
Discussion
With the changes in people’s lifestyles and dietary structures, the incidence of TB with diabetes has increased year by year. This particular combination of disease increases the body’s catabolism, causes a decrease in appetite, and affects the secretory function of the pancreas. Furthermore, the abnormal glucose metabolism of the condition is also likely to cause protein and fat metabolism disorders, leading to malnutrition and decreased cellular immune function, which further exacerbate the disease (19,20). If multidrug resistance reoccurs, it can seriously affect the treatment of patients, which not only increases the difficulty and cost of treatment, but also makes it difficult to control the disease, resulting in a reduction in cure rate and a rise in mortality (21). Therefore, early diagnosis of drug-resistant TB is the key to improving treatment efficacy. As an alternative to the culture method, molecular biology methods have been used to detect resistance mutations in rifampin and isoniazid, offering timely, accurate, and reliable detection results.
In this study, 763 TB patients’ sputum or sputum samples were tested for DNA, RNA, and acid-fast staining. The results were positive for rifampicin- and isoniazid-resistance genes. In a total of 525 patients with TB, there were 32 multi-drug resistant patients, while in a total of 207 patients with TB-DM patients, there were 15 multi-drug resistant patients. The mutation sites of ropB gene were more common with ropBS531L and ropB531 mutations, and the two sites were mostly accompanied by mutations, which is consistent with both Chinese and international studies (22). The mutation rate of ropB531 in the TB group (57.69%) was higher than that in Fujian (53.3%) and Sichuan province (55.87%), but lower than that in Guangdong (63.22%) and Guangxi (59.26%) (23). The mutation sites of the katG gene were more common with katG 315N and katG 315M, and the two sites were commonly associated with mutations, which is consistent with Chinese and international studies (24). The mutation rate of katG 315 in the TB group (315N was 82.35%, 315M was 86.76%) was higher than that in Fujian (54.35%) and lower than that in Wuhan (88.71%) (25). The mutation rate of inhA-15 locus in the TB group (16.18%) was slightly lower than that in Jiangxi (15.9%), which confirms that there are obvious regional differences in the frequency of the katG and inhA gene mutations (26). The frequency of gene mutations varies across different countries and regions (27), which may be related to the evolution of local Mycobacterium in TB. In this experiment, for the first time, the sites of multidrug resistance mutations in patients with diabetes and pulmonary TB in Zunyi were counted. Patients with simple MDR-TB are susceptible to KatG315N site mutations, while patients with diabetes and MDR-TB are more prone to inhA-15M site mutations.
Patients with TB and diabetes are mostly middle-aged and elderly men, which may be related to the characteristics of diabetes susceptibility, while women with TB and diabetes have higher blood uric acid levels. TB patients were mostly men without fixed occupations, but, because the sample size was too small, we could not rule out accidental factors. This study analyzed the correlation between relevant clinical indicators and multidrug-resistant TB infection, and found that, compared with patients with TB, the serum albumin level of patients with TB and diabetes were slightly lower, which may be associated with the fact that TB and diabetes patients cannot fully utilize glucose, which is needed to break down proteins and fats to provide calories and reduce the body’s albumin synthesis (28). However, due to the limited sample size, the relevant results are not statistically significant, and thus the sample size needs to be expanded in further research.
Acknowledgments
Funding: This study was funded by the Zunyi Science and Technology Bureau of Guizhou Province [Zunshi Kehe Support NS (019) No. 6; Zunshi Kehe Shezi (2018) No. 30].
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1368
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1368
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1368). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Guizhou Aerospace Hospital Medical Ethics Management Committee, and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Céspedes C, López L, Aguirre S, et al. Prevalence of comorbidity tuberculosis and diabetes mellitus in Paraguay, 2016 and 2017. Rev Panam Salud Publica 2019;43:e105. [Crossref] [PubMed]
- Al-Rifai RH, Pearson F, Critchley JA, et al. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS One 2017;12:e0187967. [Crossref] [PubMed]
- Ferlita S, Yegiazaryan A, Noori N, et al. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium tuberculosis. J Clin Med 2019;8:2219. [Crossref] [PubMed]
- Awad SF, Critchley JA, Abu-Raddad LJ. Epidemiological impact of targeted interventions for people with diabetes mellitus on tuberculosis transmission in India: Modelling based predictions. Epidemics 2019;30:100381. [Crossref] [PubMed]
- Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017;152:13-24. [Crossref] [PubMed]
- Degner NR, Wang JY, Golub JE, et al. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin Infect Dis 2018;66:198-205. [Crossref] [PubMed]
- Viswanathan V, Vigneswari A, Selvan K, et al. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis—a report from South India. J Diabetes Complications 2014;28:162-5. [Crossref] [PubMed]
- Lee PH, Fu H, Lai TC, et al. Glycemic control and the risk of tuberculosis: a cohort study. PLoS Med 2016;13:e1002072. [Crossref] [PubMed]
- Munir MK, Adnan M, Shabbir I, et al. Therapeutic Outcome of Pulmonary Tuberculosis in Type-2 Diabetes Patients. APMC Volume 12, Number 2 April – June 2018.
- Arriaga MB, Torres N, Araujo N, et al. Impact of the change in the antitubercular regimen from three to four drugs on cure and frequency of adverse reactions in tuberculosis patients from Brazil: A retrospective cohort study. PLoS One 2019;14:e0227101. [Crossref] [PubMed]
- Vilcheze C, Jacobs WR Jr. Resistance to isoniazid and ethionamide in mycobacterium tuberculosis: genes, mutations, and causalities. Microbiology Spectrum 2, MGM2-0014-2013.
- Gu Y, Wu C, Yu F, et al. Application of endobronchial ultrasonography using a guide sheath and electromagnetic navigation bronchoscopy in the diagnosis of atypical bacteriologically-negative pulmonary tuberculosis. Ann Transl Med 2019;7:567. [Crossref] [PubMed]
- Dai X, Wang L, Li R, et al. Different diagnostic procedures for diagnosing tuberculosis. China Public Health 2019;35:894-9.
- Wang Z. Research progress of drug-resistant tuberculosis. Infectious Disease Information 2018;31:9-23.
- Wei H, Long Y, Ling J, et al. Characteristics of resistance gene of Mycobacterium tuberculosis rifampin and isoniazid in Baise of Guangxi. Journal of Practical Medicine 2015;31:731-23.
- Jani J, Mustapha ZA, Jamal NB, et al. Whole genome sequencing data and analysis of a rifampicin-resistant Mycobacterium tuberculosis strain SBH162 from Sabah, Malaysia. Data Brief 2019;26:104445. [Crossref] [PubMed]
- Mvelase NR, Pillay M, Sibanda W, et al. rpoB Mutations Causing Discordant Rifampicin Susceptibility in Mycobacterium tuberculosis: Retrospective Analysis of Prevalence, Phenotypic, Genotypic, and Treatment Outcomes. Open Forum Infect Dis 2019;6:ofz065. [Crossref] [PubMed]
- Hameed HMA, Tan Y, Islam MM, et al. Phenotypic and genotypic characterization of levofloxacin- and moxifloxacin-resistant Mycobacterium tuberculosis clinical isolates in southern China. J Thorac Dis 2019;11:4613-25. [Crossref] [PubMed]
- Kumar NP, Moideen K, Nancy A, et al. Systemic RAGE ligands are upregulated in tuberculosis individuals with diabetes co-morbidity and modulated by anti-tuberculosis treatment and metformin therapy. BMC Infect Dis 2019;19:1039. [Crossref] [PubMed]
- Torres M, Herrera MT, Fabián-San-Miguel G, et al. The Intracellular Growth of M. tuberculosis Is More Associated with High Glucose Levels Than with Impaired Responses of Monocytes from T2D Patients. J Immunol Res 2019;2019:1462098. [Crossref] [PubMed]
- Ruesen C, Chaidir L, Ugarte-Gil C, et al. Diabetes is associated with genotypically drug-resistant tuberculosis. Eur Respir J 2020;55:1901891. [Crossref] [PubMed]
- Wang JJ, Zhou ML, Chen C, et al. Survival time and influencing factors in multidrug-resistant tuberculosis patients in Wuhan, 2006-2014). Zhonghua Liu Xing Bing Xue Za Zhi 2019;40:1409-13. [PubMed]
- Dai X. Study on the Treatment Results and Influencing Factors of Rifampicin-resistant Pulmonary Tuberculosis Patients under the Comprehensive Mode of "Medical Cooperation". 2017;01.
- Zhu Y. Evaluation of the therapeutic effect of treatment with isoniazid and rifampin on patients with isoniazid-resistant or rifampin-resistant pulmonary tuberculosis. Clin Exp Reprod Med 2016;15:1297-9.
- Guo Z, Chen X, Li X, et al. Clinical significance of direct detection of rpoB, katG and inhA resistance genes in pulmonary tuberculosis sputum smear positive specimens. Chinese and Foreign Medical 2019;38:32-5.
- Wang X, Liu J, Shen J, et al. Drug resistance of isoniazid and prothioniconamide in patients with pulmonary tuberculosis in Chongqing. Chinese Journal of Zoonoses 2019;35:173-8.
- Al-Mutairi NM, Ahmad S, Mokaddas EM. Molecular characterization of multidrug-resistant Mycobacterium tuberculosis (MDR-TB) isolates identifies local transmission of infection in Kuwait, a country with a low incidence of TB and MDR-TB. Eur J Med Res 2019;24:38. [Crossref] [PubMed]
- Vrieling F, Alisjahbana B, Sahiratmadja E, et al. Plasma metabolomics in tuberculosis patients with and without concurrent type 2 diabetes at diagnosis and during antibiotic treatment. Sci Rep 2019;9:18669. [Crossref] [PubMed]
(English Language Editor: J. Gray)