
Drug-induced chronic cough and the possible mechanism of action
Chronic cough is defined as a cough lasting for ≥8 weeks with a normal chest radiograph. The common causes of chronic cough are cough variant asthma (CVA), upper airway cough syndrome/postnasal drip syndrome (UACS/PNDs), eosinophilic bronchitis (EB), gastroesophageal reflux-related chronic cough (GERC) and atopic cough (AC). Drug-induced chronic cough is a relatively rare cause of chronic cough caused by certain drugs and resolves on its own within 1 to 4 weeks after the withdrawal of the relevant drugs, although it may linger for up to 3 months (1,2). According to the Guidelines for Diagnosis of Chronic Cough in China, patients with chronic cough should first exclude drugs as a cause. If the cough can be completely or partially relieved after drug withdrawal, the economic burden and drug adverse reactions can be reduced, and complicated examinations and the use of additional drugs can be avoided (1). It is important for diagnosis and treatment to determine the history of the patient with regard to any medications that can induce chronic cough. In addition to angiotensin-converting enzyme inhibitors (ACEIs), a relatively common drug that induces chronic cough, there are other drugs that can cause chronic cough (3-12). This article reviews the relevant drugs that may cause chronic cough and their possible mechanisms of action.
ACEIs
Cough caused by ACEIs is common in the clinical diagnosis and treatment of chronic cough (13). The biggest challenge to continuing the use of ACE inhibitors is the adverse reaction of cough. In regard to the incidence of cough after taking ACEIs, it is more common in Japan, where it reaches 20–30%, compared with Western countries, where it reaches 5–10% (13-16). Our previous study found that ACEI-associated cough accounted for approximately 3–9% of the 940 patients with chronic cough for 5 consecutive years (17). A more frequent incidence of ACEI-associated cough was noticed among elderly patients than among nonelderly patients (18). As one of the first-line treatments for hypertension (19,20), ACEIs are reportedly effective and positively indicated in patients with chronic heart failure with decreased contractility, myocardial infarction, cerebrovascular disorders, and chronic kidney disease (21-24). The above diseases are more common in elderly patients, leading to a high incidence of ACEI-associated cough among the elderly population.
Omboni et al. retrospectively analyzed twenty-three studies, with 5,794 hypertensive patients in three studies, including 1,455 post-myocardial infarction patients exposed to zofenopril at dosages of 7.5–60 mg once daily, and they found that there was a dose-effect relationship between the incidence of cough and the dosage of ACEIs. In addition, some patients experienced the relief of symptoms over time (25). In a prospective study of the frequency and characteristics of cough during ACEI treatment, Sato found that an adverse reaction of cough was observed in 20% of 176 patients with hypertension. Cough as an adverse reaction occurred at a low frequency when the medication was taken at bedtime or combined with concomitant calcium antagonists or diuretics (26). In 27,492 patients with cardiovascular disease, 1,076 patients discontinued the ACEI perindopril due to cough (3.9%) and the clinical determinants of cough were older age, female sex and the concomitant use of lipid-lowering agents (3). A study on the clinical prediction rule for ACEI-associated cough indicated that the independent multivariate predictors of cough were older age, female sex, non-African American race (with East Asians having the highest risk), no history of previous ACEI use, and a history of cough due to another ACEI (27).
The mechanism of ACEI-inducing cough mainly includes the following aspects.
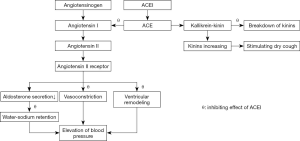
- Accumulation of bradykinin and substance P. Ramipril has been found to increase citric acid-inducing cough in guinea pigs with elevated BK and PGE2 levels in the bronchoalveolar lavage fluid (BALF). The enhancement of citric acid-induced cough caused by ramipril was reduced by the kinin B2 receptor antagonist MEN16132 (28). After taking enalapril, the concentration of substance P in the sputum of the group with a cough was significantly higher than that in the subjects without a cough (29). The cough induced by lisinopril was reversed by bradykinin B2 and NK1 receptor antagonists (30). Treatment of guinea pigs for two weeks with captopril led to an increasing cough response to inhaled citric acid, which was prevented by concomitant treatment with the bradykinin receptor antagonist icatibant. In electrophysiological studies performed in vitro, the responses of single vagal C fibers to capsaicin applied to receptive single-fiber units in the trachea were also markedly elevated after perfusion with bradykinin (31). These results indicated that bradykinin/substance P might be involved in ACEI-associated cough through the upregulation of vagal nerve excitability. The effects of ACEIs on blood pressure and cough are shown in Figure 1.
- Enhanced acetylcholine (Ach)-induced contraction of bronchial smooth muscle. Bronchial smooth muscle is dominated by the vagus nerve (parasympathetic), which mediates the contraction (cholinergic) and relaxation (noncholinergic) responses of smooth muscle (32,33). A study indicated that M2 and M3 muscarinic acetylcholine receptors were expressed in airway smooth muscle (34). Ach produces a concentration-dependent increase in bronchial smooth muscle contractions. Pretreatment with captopril significantly augments the Ach-induced contractions at each concentration. This sensitization might be responsible for the dry cough associated with ACEI captopril therapy (35). Pretreatment with aprotinin (kinin synthesis inhibitor) or heparin (inositol triphosphate, IP3 inhibitor) blocks the captopril-induced augmentation of bronchial smooth muscle contractions evoked by Ach, suggesting that the IP3 pathway in addition to bradykinin might also be involved in the process of Ach-induced bronchial smooth muscle contractions. Intracellular calcium ions play an important role in smooth muscle contraction. The influx of extracellular calcium ions into the cell causes a massive release of calcium ions in the endoplasmic reticulum, which triggers a series of changes that promote smooth muscle contraction. IP3 allows the inflow of calcium ions by releasing the calcium ions from the smooth endoplasmic reticulum, which opens store-operated Ca2+ channels (SOCCs) controlled by the calcium reservoir (36). Furthermore, captopril-induced augmentation of bronchial smooth muscle contractions is absent in calcium-free medium (35), indicating that the enhancement of bronchial smooth muscle contraction induced by acetylcholine by captopril is involved in the extracellular calcium internal flow. Another study advised caution when administering of ACEIs in asthmatic patients and those with known bronchial hyperreactivity (37). This study confirmed that ACEI-associated cough in asthmatic patients might be correlated with bronchial hyperreactivity.
- Gene polymorphisms. A genome-wide association study (GWAS) of ACEI-associated cough found that differences in the introns of the Kv channel interacting protein 4 (KCNIP4) on chromosome 4 may modulate the risk of ACEI-induced cough (38). GWASs in mice have shown a link between KCNIP4 and airway hyperresponsiveness (AHR), which has been confirmed in studies of human asthma and AHR (39). In addition, ABO genetic polymorphisms have recently been associated with angiotensin-converting enzyme (ACE) activity and inflammation, which play a critical role in the pathogenic mechanism of ACE inhibitor-induced cough. A study of ABO genetic polymorphisms in 450 essential hypertensive Chinese patients treated with enalapril indicated that the rs495828 polymorphism was associated with enalapril-induced cough. The results for the rs8176740 polymorphism were significant in the female subgroup (40). At the same time, there was a strong interaction between rs495828 and rs8176746. Bradykinin II (B2) receptor polymorphism was also associated with ACEI-associated cough, while the B2-9 allele reduced ACE activity and caused a cough (41). In addition, the bradykinin II receptor gene rs8016905 was also associated with ACEI-induced cough (42), suggesting the importance of bradykinin in the pathogenesis of ACEI-associated cough. Furthermore, studies on NK-2 receptor gene polymorphisms found that Gly231Glu was negatively correlated with cough (43).
Angiotensin receptor blockers (ARBs)
ARBs can be used as an alternative treatment for patients with ACEI drug intolerance because they cause a lower incidence of cough, are better tolerated and obtain similar or better antihypertensive effects (4). Cough was the most frequent reason to stop ACEI treatment according to a study of 27,492 patients taking ACEIs, and the rate of switching to ARBs as a second-line alternative due to cough was approximately 4% (3). ARBs carry a risk of cough similar to that of a placebo/diuretics, and it is significantly lower than that related to ACEIs. It is necessary to be alert to the occurrence of cough during treatment with ARBs because the incidence of ARB-associated cough was found to be as high as 20% in early studies (44). As mentioned above, elevated bradykinin levels were thought to be connected to ACEI-associated cough and ARBs could obtain similar bradykinin levels as ACEI due to the reduced metabolism by ACE and neutral endopeptidase, which might be one of the causes of ARB-associated cough (45,46). ACEI-associated cough might be influenced by multiple factors. In addition to substance P, ACEIs might also affect ACE function in susceptible patients, and an increase in the level of kinin might be caused by a gene polymorphism of the bradykinin receptor (42,47). Therefore, the incidence of ARB-associated cough is significantly lower than that of ACEI-associated cough in this specific population.
Opioids
Cough is one of the most common complications of opioids. According to previous studies, the incidence of opioid-induced cough was approximately 28–66%, although lidocaine, propofol and other drugs could partially inhibit this side effect (48,49). The following aspects may be involved in the mechanism underlying opioid-induced cough. First, opioids may inhibit central sympathetic outflow and, in turn, activate the vagus nerve. The enhancement of the parasympathetic nervous system is suggested to cause cough and bronchoconstriction (5,50). Second, the pulmonary chemoreflex may be another likely mechanism. Studies have shown that fentanyl and morphine may cause coughing by promoting the release of histamine, which could enhance the excitability of rapidly adapting receptors (RARs) (51,52). Third, opioid receptors exist in the upper pulmonary mucosa and opioid-induced tracheal and bronchial smooth muscle constriction may trigger the cough reflex (53). Moreover, opioid-induced muscle rigidity, which can cause sudden adduction of the vocal cords or supra-glottic obstruction by soft tissue, may be a possible mechanism (53,54). Former smokers are more likely than current smokers to experience cough, according to a study about the association of fentanyl-induced cough and smoking (55). Desensitization of the cough receptors within the airway epithelium and increased airway mucus secretion covering cough receptors after long-term tobacco smoking may be a possible mechanism for the suppression of cough sensitivity in smoking patients (56,57).
Statins
The statins 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors can effectively reduce plasma low-density lipoprotein (LDL) levels and cholesterol levels, improving the incidence of cardiovascular events and mortality (58,59). Patients diagnosed with hypercholesterolemia and undergoing treatment with statins can experience a series of side effects including coughing, and all the symptoms achieve remission after discontinuation of the treatment for 7–15 days, although the improvement rate of cough only reaches 91.7% (60). According to an analysis from 2004 to 2012 using the Australian database of Adverse Event Notifications (DAEN), the Canada Vigilance Adverse Reaction Online Database and the United States Food and Drug Administration Adverse Event Reporting System (AERS), cough as an adverse reaction is not uncommon in the clinical application of statins. There might be two possible explanations for statin-induced cough. First, the administration of statins could induce PG synthase and increase prostacyclin production, which might cause coughing. Second, statins could stabilize the mRNA of endothelial nitric oxide (NO) synthase, leading to enhanced enzyme expression and NO generation that could increase cough reflex sensitivity (61). According to the case report, statins have interstitial lung disease as a side effect, which could manifest as coughing, but the specific mechanism leading to lung injury is unknown (62). According to three major international adverse event databases, 88% of patients with statin-induced cough lacked lung injury, suggesting that the mechanism underlying statin-induced cough is inconsistent with lung injury (61). The hypothesis is that the mechanism underlying statin-induced bronchial hypersensitivity might be related to the inhibition of HMG-CoA reductase, although further studies are needed to confirm the connection (6). In addition, Liesmaa et al. found that lovastatin up-regulates bradykinin type II receptor expression on human coronary artery endothelial cells in vitro (63), which is related to the mechanism underlying ACEI-associated cough (41). This finding might also explain why cough is more likely to occur when ACEIs are combined with lipid-lowering drugs, although the exact mechanism requires further research.
Rare case reports of other drugs
Omeprazole
GERC is one of the common causes of chronic cough. Empirical treatment of suspected GERC is recommended in guidelines from several countries, and the most common treatment is a proton pump inhibitor (PPI) combined with a gastroprokinetic agent (1,2,64,65). Prescription information for omeprazole and pantoprazole indicates that cough symptoms were observed in the trial population (66,67). In addition, case reports suggested that cough induced by omeprazole depended on the drug concentration and is mainly related to the plasma concentration of omeprazole. Therefore, many scholars maintain that cough may be a side effect of PPIs (8). The mechanism underlying omeprazole-induced cough is still unclear, and whether it has a direct pharmacological effect on the receptors involved in the cough reflex remains to be determined.
Leflunomide (LFM)
LFM is a disease-modifying antirheumatic drug. The long-term use of LFM affects multiple systems. The respiratory side effects included bronchitis (7%), bronchospasm and increased cough (3%), respiratory tract infection (15%), pharyngitis (3%), pneumonia (2%), rhinitis (2%) and sinusitis (2%) (68). The cough caused by LFM should be considered for two reasons. First, infection including tuberculosis after immunosuppression might account for the occurrence of cough (69). Second, a cough may be directly caused by LFM, although the mechanism needs to be further explored.
Interferon (IFN) and ribavirin
Dry cough in chronic hepatitis C patients treated with interferon-α monotherapy might result from the indirect effects of IFN immunological pathways (9). After IFN treatment, soluble interleukin-2 (IL-2) receptor levels are significantly increased in patients with chronic hepatitis C (70). It is interesting that plasma soluble IL-2 receptor levels are significantly elevated in patients with acute asthma attacks (71). The association supports the possibility of an immune mechanism in the pathogenesis of interferon-related cough. The combination of pegylated interferon and ribavirin has become standard therapy for chronic hepatitis C infection (72). Dicpinigaitis found that 4 patients without a history of respiratory symptoms developed chronic cough temporally related to the initiation of therapy with pegylated interferon and ribavirin for chronic hepatitis C infection. The cough resolved after the completion of therapy. Capsaicin cough challenge testing was performed to measure the patients’ cough reflex sensitivity. In all patients, cough reflex sensitivity was significantly enhanced during treatment compared to 1 month after the completion of therapy (73). Previous studies have observed that cough occurs more commonly in patients receiving the combination of interferon and ribavirin compared to those receiving interferon alone (74,75). The mechanism by which ribavirin might induce cough remains a matter of speculation, although it might enhance UACS/PNDs or gastroesophageal reflux (2), which are known causes of chronic cough. Alternatively, ribavirin might directly stimulate coughing through the activation of the transient receptor potential (TRP) V1 or A1 ion channels, the importance of which in the induction of cough has recently been elucidated (76,77).
Sitagliptin
Sitagliptin is a highly selective oral dipeptidyl peptidase-4 (DPP IV) inhibitor that inhibits the breakdown of incretins such as glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide (78), which is used as a monotherapy or as a component of combination therapy for the treatment of patients with type 2 diabetes (79). In a placebo-controlled trial assessing the efficacy and tolerability of sitagliptin in patients with type 2 diabetes mellitus who were inadequately controlled on metformin alone, the incidence of cough as a side effect was reported to be higher in the sitagliptin group compared with the placebo group (80). A case series described 15 patients intolerant to sitagliptin, where 13 of them reported cough with other respiratory symptoms such as rhinorrhea or dyspnea/wheeze. All of them had underlying allergic rhinitis, and the frequency was significantly higher than that in sitagliptin-tolerant patients. Nasal and inhaled glucocorticoids may control the underlying allergic inflammation and abrogate this new sitagliptin-induced pharmacological syndrome. Potential mucosal and central nervous system mechanisms include disruption of neuropeptides and/or cytokines that rely on DPP IV for activation or inactivation, and T cell dysfunction (81). However, the risk of cough was not evident in any other RCTs or pooled analyses comparing sitagliptin and placebo (82-85). Placebo controlled trials with other DPP IV inhibitors, including linagliptin, saxagliptin and vildagliptin, also did not report any increased risk of cough (86-88). Based on these studies, many scholars maintained that there was a slight increased risk of nasopharyngitis with the use of sitagliptin compared with placebo. However, sufficient evidence is not available to associate an increased risk of acute cough, chronic cough or lower respiratory tract infection with sitagliptin or any other DPP-4 inhibitor (89). These findings collectively suggested the risk of sitagliptin-induced cough is not clear, which need more researches to be determined.
Summary
Although drug-induced chronic cough is not one of the common causes of chronic cough, it accounts for a proportion of the incidence. More attention should be focused on the patient’s medication history and identifying the drugs that can induce cough in a timely manner. If the cough occurs after taking the medicine, a suspected diagnosis of drug-induced cough should be established. If the cough resolution occurs within 1 to 4 weeks after drug withdrawal, it would be considered as a side effect of the medication. To support the clinical diagnosis and treatment of chronic cough, we should investigate the newest research on drug-induced cough and identify relevant drugs that may cause chronic cough. For unexplained chronic cough, after excluding CVA, UACS, EB and other common causes of chronic cough, the possibility of drug-induced chronic cough should be considered.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 81770097), the Project of Science and Technology Commission of Shanghai Municipality (No. 20ZR1451500), the Fund of Shanghai Youth Talent Support Program, and the Fund of Shanghai Municipal Health Commission for Excellent Young Scholars (No. 2018YQ01).
Footnote
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-819
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-819). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Group CMAoRDAS. Guidelines for the diagnosis and treatment of coughing (2015). Zhonghua Jie He He Hu Xi Za Zhi 2016;39:323-54.
- Irwin RS, Baumann M, Bolser D, et al. Diagnosis and Management of Cough Executive Summary : ACCP Evidence-Based Clinical Practice Guidelines. Chest 2006;129:1S-23S. [Crossref] [PubMed]
- Brugts JJ, Arima H, Remme W, et al. The incidence and clinical predictors of ACE-inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol 2014;176:718-23. [Crossref] [PubMed]
- Mancia G, Schumacher H. Incidence of adverse events with telmisartan compared with ACE inhibitors: evidence from a pooled analysis of clinical trials. Patient Prefer Adherence 2012;6:1-9. [PubMed]
- Kim JE, Min S, Chae Y, et al. Pharmacological and nonpharmacological prevention of fentanyl-induced cough: a meta-analysis. J Anesth 2014;28:257-66. [Crossref] [PubMed]
- Psaila M, Fsadni P, Montefort S. Chronic cough as a complication of treatment with statins: a case report. Ther Adv Respir Dis 2012;6:243-6. [Crossref] [PubMed]
- Verma S, Mishra A, Jaiswal A. Leflunomide-induced chronic cough in a rheumatoid arthritis patient with pulmonary tuberculosis. BMJ Case Rep 2013;2013:bcr2012008373. [Crossref] [PubMed]
- Reiche I, Troger U, Martens-Lobenhoffer J, et al. Omeprazole-induced cough in a patient with gastroesophageal reflux disease. Eur J Gastroenterol Hepatol 2010;22:880-2. [Crossref] [PubMed]
- Isler M, Akhan G, Bardak Y, et al. Dry cough and optic neuritis: two rare complications of interferon alpha treatment in chronic viral hepatitis. Am J Gastroenterol 2001;96:1303-4. [PubMed]
- Irwin RS, French CL, Chang AB, et al. Classification of Cough as a Symptom in Adults and Management Algorithms: CHEST Guideline and Expert Panel Report. Chest 2018;153:196-209. [Crossref] [PubMed]
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136. [Crossref] [PubMed]
- Rhee CK, Jung JY, Lee SW, et al. The Korean Cough Guideline: Recommendation and Summary Statement. Tuberc Respir Dis (Seoul) 2016;79:14-21. [Crossref] [PubMed]
- Visser LE, Stricker BH, van der Velden J, et al. Angiotensin converting enzyme inhibitor associated cough a population-based case-control study. J Clin Epidemiol 1995;48:851-7. [Crossref] [PubMed]
- Bangalore S, Kumar S, Messerli F. Angiotensin-Converting Enzyme Inhibitor Associated Cough: Deceptive Information from the Physicians' Desk Reference. Am J Med 2010;123:1016-30. [Crossref] [PubMed]
- Saruta T, Arakawa K, Iimura O, et al. Difference in the incidence of cough induced by angiotensin converting enzyme inhibitors a comparative study using imidapril hydrochloride and enalapril maleate. Hypertens Res 1999;22:197-202. [Crossref] [PubMed]
- Sadanaga T, Yoshimura M, Sakamoto T, et al. Enalapril induced cough is associated with nonsevere heart failure. Int J Cardiol 2009;135:275-6. [Crossref] [PubMed]
- Yu L, Wei W, Lv H, et al. Changes in the Spectrum and frequency of causes for chronic cough: a retrospective analysis. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:414-7. [PubMed]
- Wei W, Yu L, Lv H, et al. Comparison of cause distribution between elderly and non-elderly patients with chronic cough. Respiration 2009;77:259-64. [Crossref] [PubMed]
- Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014;37:253-390. [Crossref] [PubMed]
- Cifu AS, Davis AM. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. JAMA 2017;318:2132-4. [Crossref] [PubMed]
- Group CTS. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N Engl J Med 1987;316:1429-35. [Crossref] [PubMed]
- Kober L, Torp-Pedersen C, Carlsen J, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med 1995;333:1670-6. [Crossref] [PubMed]
- Group PC. Randomized trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41. [Crossref] [PubMed]
- Maschio G, Alberti D, Locatelli F, et al. Angiotensin-converting enzyme inhibitors and kidney protection: the AIPRI trial. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study Group. J Cardiovasc Pharmacol 1999.33-discussion S41-3. [PubMed]
- Omboni S, Borghi C. Zofenopril and incidence of cough: a review of published and unpublished data. Ther Clin Risk Manag 2011;7:459-71.
- Sato A, Fukuda S. A prospective study of frequency and characteristics of cough during ACE inhibitor treatment. Clin Exp Hypertens 2015;37:563-8. [Crossref] [PubMed]
- Morimoto T, Gandhi TK, Fiskio JM, et al. Development and Validation of a Clinical Prediction Rule for Angiotensin-converting Enzyme Inhibitor-induced Cough. J Gen Intern Med 2004;19:684-91. [Crossref] [PubMed]
- Cialdai C, Giuliani S, Valenti C, et al. Differences between zofenopril and ramipril, two ACE inhibitors, on cough induced by citric acid in guinea pigs: role of bradykinin and PGE2. Naunyn Schmiedebergs Arch Pharmacol 2010;382:455-61. [Crossref] [PubMed]
- Tomaki M, Ichinose M, Miura M, et al. Angiotensin converting enzyme (ACE) inhibitor-induced cough and substance P. Thorax 1996;51:199-201. [Crossref] [PubMed]
- Cinelli E, Bongianni F, Pantaleo T, et al. The cough reflex is upregulated by lisinopril microinjected into the caudal nucleus tractus solitarii of the rabbit. Respir Physiol Neurobiol 2015;219:9-17. [Crossref] [PubMed]
- Fox AJ, Lalloo UG, Belvisi MG, et al. Bradykinin-evoked sensitization of airway sensory nerves: A mechanism for ACE-inhibitor cough. Nat Med 1996;2:814-7. [Crossref] [PubMed]
- Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respir Physiol 2001;125:113-27. [Crossref] [PubMed]
- Canning BJ, Woo A, Mazzone SB. Neuronal modulation of airway and vascular tone and their influence on nonspecific airways responsiveness in asthma. J Allergy (Cairo) 2012;2012:108149. [Crossref] [PubMed]
- Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther 2003;98:59-69. [Crossref] [PubMed]
- Agrawal N, Akella A, Deshpande SB. Captopril augments acetylcholine-induced bronchial smooth muscle contractions in vitro via kinin-dependent mechanisms. Indian J Exp Biol 2016;54:365-9. [PubMed]
- Barrett KE, Boitano S, Barman SM, et al. Regulation of extracellular fluid composition and volume. In: Ganong’s Review of Medical Physiology. 23rd ed. New York: McGraw Hill, 2009:665.
- Kaufman J, Casanova JE, Riendl P, et al. Bronchial hyperreactivity and cough due to angiotensin-converting enzyme inhibitors. Chest 1989;95:544-8. [Crossref] [PubMed]
- Mosley JD, Shaffer CM, Van Driest SL, et al. A genome-wide association study identifies variants in KCNIP4 associated with ACE inhibitor-induced cough. Pharmacogenomics J 2016;16:231-7. [Crossref] [PubMed]
- Himes BE, Sheppard K, Berndt A, et al. Integration of mouse and human genome-wide association data identifies KCNIP4 as an asthma gene. PLoS One 2013;8:e56179. [Crossref] [PubMed]
- Luo JQ, He FZ, Luo ZY, et al. Rs495828 polymorphism of the ABO gene is a predictor of enalapril-induced cough in Chinese patients with essential hypertension. Pharmacogenet Genomics 2014;24:306-13. [PubMed]
- Moholisa RR, Rayner BR, Patricia Owen E, et al. Association of B2 receptor polymorphisms and ACE activity with ACE inhibitor-induced angioedema in black and mixed-race South Africans. J Clin Hypertens (Greenwich) 2013;15:413-9. [PubMed]
- Mas S, Gasso P, Alvarez S, et al. Pharmacogenetic predictors of angiotensin-converting enzyme inhibitor-induced cough: the role of ACE, ABO, and BDKRB2 genes. Pharmacogenet Genomics 2011;21:531-8. [PubMed]
- Kim TB, Oh S, Park H, et al. Polymorphisms in the neurokinin-2 receptor gene are associated with angiotensin-converting enzyme inhibitor-induced cough. J Clin Pharm Ther 2009;34:457-64. [Crossref] [PubMed]
- Caldeira D, David C, Sampaio C. Tolerability of Angiotensin-Receptor Blockers in Patients with Intolerance to Angiotensin-Converting Enzyme Inhibitors. Am J Cardiovasc Drugs 2012;12:263-77. [Crossref] [PubMed]
- Tomiyama H, Motobe K, Zaydun G, et al. Insulin sensitivity and endothelial function in hypertension: A comparison of temocapril and candesartan. Am J Hypertens 2005;18:178-82. [Crossref] [PubMed]
- Campbell DJ, Krum H, Esler MD. Losartan increases bradykinin levels in hypertensive humans. Circulation 2005;111:315-20. [Crossref] [PubMed]
- Mukae S, Itoh S, Aoki S, et al. Association of polymorphisms of the renin-angiotensin system and bradykinin B2 receptor with ACE-inhibitor-related cough. J Hum Hypertens 2002;16:857-63. [Crossref] [PubMed]
- Shuying L, Ping L, Juan N, et al. Different interventions in preventing opioid-induced cough: a meta-analysis. J Clin Anesth 2016;34:440-7. [Crossref] [PubMed]
- Firouzian A, Emadi S, Baradari A, et al. Can low dose of propofol effectively suppress fentanyl-induced cough during induction of anaesthesia? A double blind randomized controlled trial. J Anaesthesiol Clin Pharmacol 2015;31:522-5. [Crossref] [PubMed]
- Agarwal A, Azim A, Ambesh S, et al. Salbutamol, beclomethasone or sodium chromoglycate suppress coughing induced by iv fentanyl. Can J Anaesth 2003;50:297-300. [Crossref] [PubMed]
- Kamei J, Nakanishi Y, Asato M, et al. Fentanyl enhances the excitability of rapidly adapting receptors to cause cough via the enhancement of histamine release in the airways. Cough 2013;9:3. [Crossref] [PubMed]
- Withington D E, Patrick J A, Reynolds F. Histamine Release by Morphine and Diamorphine in Man. Anaesthesia 1993;48:26-9. [Crossref] [PubMed]
- Yasuda I, Hirano T, Yusa T, et al. Tracheal constriction by morphine and by fentanyl in man. Anesthesiology 1978;49:117-9. [Crossref] [PubMed]
- Phua WT, Teh BT, Jong W, et al. Tussive effect of a fentanyl bolus. Can J Anaesth 1991;38:330-4. [Crossref] [PubMed]
- Jung HJ, Kim JB, Im KS, et al. Effects of a priming dose of fentanyl during anaesthesia on the incidence and severity of fentanyl-induced cough in current, former and non-smokers. J Int Med Res 2011;39:2379-84. [PubMed]
- Dicpinigaitis PV. Cough reflex sensitivity in cigarette smokers. Chest 2003;123:685-8. [PubMed]
- Dicpinigaitis PV, Sitkauskiene B, Stravinskaite K, et al. Effect of smoking cessation on cough reflex sensitivity. Eur Respir J 2006;28:786-90. [Crossref] [PubMed]
- Rosenson RS, Tangney C. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA 1998;279:1643-50. [Crossref] [PubMed]
- Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004;109:III50-7. [Crossref] [PubMed]
- Pascual Cruz M, Chimenos Küstner E, García Vicente JA, et al. Adverse side effects of statins in the oral cavity. Med Oral Patol Oral Cir Bucal 2008;13:E98-101. [PubMed]
- Carnovale C, Pellegrino P, Perrone V, et al. Establishing the correlation between statins and cough: case series report and analysis of adverse drug reactions in the international databases. Eur J Clin Pharmacol 2014;70:1529-31. [Crossref] [PubMed]
- Kim S, Kim S, Yoon D, et al. A Case of Statin-Induced Interstitial Pneumonitis due to Rosuvastatin. Tuberc Respir Dis (Seoul) 2015;78:281-5. [Crossref] [PubMed]
- Liesmaa I, Kokkonen JO, Kovanen PT, et al. Lovastatin induces the expression of bradykinin type 2 receptors in cultured human coronary artery endothelial cells. J Mol Cell Cardiol 2007;43:593-600. [Crossref] [PubMed]
- Morice AH, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004;24:481-92. [Crossref] [PubMed]
- Kohno S, Ishida T, Uchida Y, et al. The Japanese Respiratory Society guidelines for management of cough. Respirology 2006;11 Suppl 4:S135. [PubMed]
- Prescribing. information. Prilosec (omeprazole). Astra Zeneca (2008). Available online: Accessed April 20 2009.http://www.astrazeneca-us.com/pi/Prilosec.pdf.
- Prescribing information. Protonix (pantoprazole). Wyeth Pharmaceuticals (2008). Available online: http://www.wyeth.com/content/showlabeling.asp?id=135
- Available online: http://www.drugs.com/sfx/leflunomide-side-effects.html
- Yoo HG, Yu HM, Jun JB, et al. Risk factors of severe infections in patients with rheumatoid arthritis treated with leflunomide. Mod Rheumatol 2013;23:709-15. [Crossref] [PubMed]
- Sugimura T, Motomura S, Sakai H, et al. Increased serum soluble IL-2 receptor levels following interferon therapy in patients with chronic hepatitis C. Hepatogastroenterology 1999;46:1827-30. [PubMed]
- Shi HZ, Sun JJ, Pan HL, et al. Alterations of T-lymphocyte subsets, soluble IL-2 receptor, and IgE in peripheral blood of children with acute asthma attacks. J Allergy Clin Immunol 1999;103:388-94. [Crossref] [PubMed]
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med 2006;355:2444-51. [Crossref] [PubMed]
- Dicpinigaitis PV, Weiner F. Chronic cough associated with interferon/ribavirin therapy for hepatitis C. J Clin Pharm Ther 2011;36:416-8. [Crossref] [PubMed]
- Brok J, Gluud LL, Gluud C. Effects of adding ribavirin to interferon to treat chronic hepatitis C infection: a systematic review and meta-analysis of randomized trials. Arch Intern Med 2005;165:2206-12. [Crossref] [PubMed]
- Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009;360:1839-50. [Crossref] [PubMed]
- McLeod RL, Correll C, Jia Y, et al. TRPV1 antagonists as potential antitussive agents. Lung 2008;186:S59-65. [Crossref] [PubMed]
- Taylor-Clark TE, Nassenstein C, McAlexander M, et al. TRPA1: a potential target for anti-tussive therapy. Pulm Pharmacol Ther 2009;22:71-4. [Crossref] [PubMed]
- Kim NH, Yu T, Lee DH. The nonglycemic actions of dipeptidyl peptidase-4 inhibitors. Biomed Res Int 2014;2014:368703. [Crossref] [PubMed]
- Richter B, Bandeira-Echtler E, Bergerhoff K, et al. Dipeptidyl peptidase-4 (Dpp-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008;2:CD006739. [Crossref] [PubMed]
- Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006;29:2638-43. [Crossref] [PubMed]
- Baraniuk JN, Jamieson MJ. Rhinorrhea, cough and fatigue in patients taking sitagliptin. Allergy Asthma Clin Immunol 2010;6:8. [Crossref] [PubMed]
- Williams-Herman D, Engel SS, Round E, et al. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord 2010;10:7. [Crossref] [PubMed]
- Engel SS, Round E, Golm GT, et al. Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther 2013;4:119-45. [Crossref] [PubMed]
- Arjona Ferreira JC, Corry D, Mogensen CE, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis 2013;61:579-87. [Crossref] [PubMed]
- Ahrén B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014;37:2141-8. [Crossref] [PubMed]
- Cai L, Cai Y, Lu ZJ, et al. The efficacy and safety of vildagliptin in patients with type 2 diabetes: a meta-analysis of randomized clinical trials. J Clin Pharm Ther 2012;37:386-98. [Crossref] [PubMed]
- Lehrke M, Marx N, Patel S, et al. Safety and Tolerability of Linagliptin in Patients With Type 2 Diabetes: A Comprehensive Pooled Analysis of 22 Placebo-controlled Studies. Clin Ther 2014;36:1130-46. [Crossref] [PubMed]
- Doucet J, Chacra A, Maheux P, et al. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr Med Res Opin 2011;27:863-9. [Crossref] [PubMed]
- Dicpinigaitis P, Satia I, Ferguson N. Falsely Accused? Insufficient Evidence to Conclude that Sitagliptin is a Cause of Chronic Cough. Lung 2020;198:271-3. [Crossref] [PubMed]


