Palliative radiotherapy: past, present and future—where do we go from here?
Editor’s note:
“Palliative Radiotherapy Column” features in articles emphasising the critical role of radiotherapy in palliative care. Chairs to the columns are Dr. Edward L.W. Chow from Odette Cancer Centre, Sunnybrook Health Sciences Centre in Toronto and Dr. Stephen Lutz from Blanchard Valley Regional Cancer Center in Findlay, gathering a group of promising researchers in the field to make it an excellent column. The column includes original research manuscripts and timely review articles relating to palliative radiotherapy, and editorials and commentaries on recently published trials and studies as well.
Introduction
Radiotherapy has been used for palliating symptoms of cancer since soon after its discovery in the 1800’s (1). While the radiation oncology specialty has incompletely embraced its usefulness in palliative oncology, this treatment modality has proven itself to be a cost-effective and time-efficient intervention that is associated with a low toxicity profile. Radiotherapy can relieve symptoms due to either primary or metastatic tumors, including common manifestations of cancer such as pain, obstruction, bleeding, and neurologic symptoms. While the complexity of palliative radiotherapy has increased with that advent of newer technologies and the need to collaborate with other involved specialties, the common sense goals of its delivery remain a good chance for symptom relief with a limited risk of side effects. Here we preview a series of upcoming papers in this and future issues of the Annals of Palliative Medicine that will highlight the benefits, controversies, and future promise of palliative radiotherapy for end-of-life oncology care.
Prognostic factors and life expectancy
Optimal palliative oncology care requires both an accurate estimation of life expectancy as well as a determination of whether palliative treatments are to be delivered concurrently with curative-intent therapy or with palliative intent, only. Several factors complicate the survival prognostication of cancer patients, including patient factors such as co-morbid illnesses, disease-related factors such as tumor stage and histology, and psychological factors such as the desire of caregivers to maintain a hopeful outlook. This third factor might explain why physicians often describe an unrealistically optimistic prognosis, commonly overestimating survival by a factor of three or more (2-6). Drawbacks of these inaccurately lengthy prognostications include unrealistic patient expectations and a tendency toward overly aggressive interventions (7-9). Prognostic models that assess multiple factors and predict survival in patients receiving palliative radiotherapy for advanced cancer have been developed and tested, giving hope that treatment decisions will come to more closely align with realistic survival estimates (10,11).
Hypofractionated palliative radiotherapy
Patient and caregiver convenience dictate that palliative radiotherapy treatment courses are given in as short a time period as will allow their effectiveness, especially in those patients where prognosis suggests a short lifespan. While curative treatment regimens have evolved to deliver daily fractions of 1.8 to 2.0 Gy for a total of 40 to 80 Gy, palliative radiotherapy can be effective with dose regimens of 8 to 30 Gy in 1 to 10 fractions. Beyond survival estimates, factors that influence palliative radiotherapy fractionation include: patient performance status, comorbidities, and transportation capabilities; tumor factors such as number, location, and behavior of local and metastatic lesions; and, radiotherapy toxicity risks, taking into account any previous radiotherapy to the same anatomic site as well as other potential combined toxicities caused by other modalities of treatment that have been given. Multiple studies have yielded information about hypofractionated regimens that provide palliative relief for a myriad of clinical circumstances (Table 1) (12).
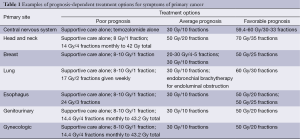
Full table
Radiotherapy dose fractionation for bone metastases
The palliation of painful bone metastases with short courses of palliative radiotherapy remains one of the most striking examples of the value of this type of treatment to end-of-life cancer patients. Bone metastases are a very common manifestation of malignancy, and radiotherapy provides partial pain relief in 60-80% and complete pain relief in 30-50% of patients within days to weeks after the initiation of therapy (13). The American Society for Radiation Oncology (ASTRO) Bone Metastases Guidelines concluded that pain relief is equivalent with fractionation regimens of 30 Gy in ten fractions, 24 Gy in six fractions, 20 Gy in five fractions, or a single 8 Gy fraction (14). Though retreatment rates may be higher in those who receive a single fraction, a second course of therapy can be expected to provide a reasonable rate of pain relief (15). Still, practice patterns within the US have not fully come to match the available data and guideline recommendations (16,17).
Radiotherapy management of complex spine lesions
Malignant epidural spinal cord compression (MESCC) is an important special circumstance of bone metastases because its effects may include neurologic compromise in addition to pain. Manifestations of this clinical condition may include loss of sensation, paralysis, and incontinence of bowel or bladder (18). Management of MESCC requires prompt diagnosis, initiation of corticosteroids to diminish edema, multidisciplinary evaluation of the potential benefits and risks of surgical decompression, and radiotherapy either as the main treatment type or as an adjuvant in the post-operative setting (19,20). The proper dose fractionation scheme for patients with MESCC remains an active topic of investigation (21-24).
Anticipation and management of palliative radiotherapy side effects
Every radiotherapy intervention can be associated with acute or late toxicity, with factors that influence those risks including volume of tissue irradiated, total radiation dose, dose per fraction, toxicities of other treatment modalities, and the radiosensitivity of normal adjacent tissues (25). As opposed to circumstances in which radiation is delivered with curative intent, palliative radiotherapy does not necessarily require complete tumor ablation or treatment of all known disease to provide symptom relief (26). In fact, lower total doses limit acute toxicity and allow for improved convenience through a shorter treatment course. In general, palliative radiotherapy doses are delivered with larger fraction sizes than are used for curative intent courses. These hypofractionated courses may provide the benefit of earlier symptoms response but at the cost of a greater risk of late side effects (27). Still, risks of late side effects can be minimized by limiting the total biologic equivalent dose of the palliative radiotherapy regimen delivered. Also, given that late side effects commonly occur months to years following completion of treatment, the sad truth is that the implications of this type risk may be irrelevant for palliative patients who may not live long enough to face late effects (25).
Palliative radiotherapy guidelines and quality measures
Documented disparities in palliative radiotherapy treatment approaches as well as resource limitations in the face of growing patient needs have combined to lead for calls to produce guidelines and quality measures. Whereas guidelines derive clinical treatment recommendations from available high-quality literature, quality measures are meant to both endorse standards and measure performance of individual caregivers and health care systems (28). Three of the first six ASTRO treatment guidelines dealt with palliative care scenarios, confirming the importance of the topic to the oncology community, at large (29).
The recognition of variable practice patterns in the radiotherapeutic treatment of bone metastases led to the completion of the ASTRO Bone Metastases Guidelines (14,30). The National Quality Forum accepted a submission based upon this guideline as its first quality measure for the evaluation of radiation oncology practices (31). Furthermore, the American Board of Internal Medicine initiative entitled Choosing Wisely chose as one of its first radiation oncology recommendations, “don’t routinely use extended fractionation schemes (>10 fractions) for palliation of bone metastases”. The successful progression of the initial bone metastases fractionation question into a treatment guideline, a quality measure, and a recommendation to all radiation oncologists and patients in the Choosing Wisely campaign suggests that further palliative radiotherapy initiatives will be well received.
Future directions
In addition to further palliative radiotherapy guidelines and quality measures, future initiatives will need to help the radiation oncology specialty to continue to balance the technological advances in treatment delivery with the special needs of dying patients. For instance, the promise for improved tumor control with diminished toxicity is great for newer treatment types such as stereotactic radiosurgery for brain metastases and stereotactic body radiotherapy for clinical conditions such as primary lung cancer or metastases in the spine, liver, or lung. Still, the costs of this technology may further strain the resource limitations associated with the increased number of patients expected as 78 million “Baby Boomers” reach the age where their cancer incidence rises. Clearly, specialized treatments such as those offered by radiation oncologists will need to be better coordinated with overall palliative care goals of this patient group. One study, in particular, exemplified the usefulness of early palliative care intervention for patients with newly diagnosed, locally advanced non-small cell lung cancer. Those who were randomly assigned to early palliative care consultation in addition to stand care were shown to have improved quality of life, lower rates of depression, and longer survival (32).
Furthermore, just as academic radiation oncology departments have been subdivided into teams that care for patients with specific types of diagnoses, so too must they consider the formation of palliative radiotherapy services. Some centers have begun to pioneer this type of approach in an effort to optimize the coordinated delivery of end-of-life oncology care for patients consulted for palliative radiotherapy. Still other centers have tried to establish ‘rapid response’ clinical pathways that optimize throughput of palliative radiotherapy patients to allow them to undergo consultation, simulation, and treatment delivery in one visit (33).
Finally, in spite of the number of patients who receive palliative oncology care in the United States each year, as well as the severity of their symptoms, there are a paucity of research trials devoted to this topic. While the Radiation Therapy and Oncology Group (RTOG) has completed and published several trials regarding the most appropriate care of patients with bone and brain metastases, future research will require increased interdisciplinary input into trial designs, a greater number of validated quality of life instruments, and a more creative manner to collect follow up data in a setting where missing data points are common as a result of declining function or death of the patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jones J. A Brief History of Palliative Radiation Oncology. In: Lutz S, Chow E, Hoskin P. eds. Radiation Oncology in Palliative Cancer Care. West Sussex: Wiley-Blackwell, 2013.
- Christakis NA, Lamont EB. Extent and determinants of error in physicians’ prognoses in terminally ill patients: prospective cohort study. West J Med 2000;172:310-3. [PubMed]
- Chow E, Harth T, Hruby G, et al. How accurate are physicians’ clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin Oncol (R Coll Radiol) 2001;13:209-18. [PubMed]
- Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ 2000;320:469-72. [PubMed]
- Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003;327:195-8. [PubMed]
- Oxenham D, Cornbleet MA. Accuracy of prediction of survival by different professional groups in a hospice. Palliat Med 1998;12:117-8. [PubMed]
- Krishnan M, Temel JS, Wright AA, et al. Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol 2013;11:68-74. [PubMed]
- Mack JW, Chen K, Boscoe FP, et al. Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol 2013;31:2569-79. [PubMed]
- Meeuse JJ, van der Linden YM, van Tienhoven G, et al. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life: results from the Dutch Bone Metastasis Study. Cancer 2010;116:2716-25. [PubMed]
- Chow E, Abdolell M, Panzarella T, et al. Validation of a predictive model for survival in metastatic cancer patients attending an outpatient palliative radiotherapy clinic. Int J Radiat Oncol Biol Phys 2009;73:280-7. [PubMed]
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: the TEACHH model. Cancer 2014;120:134-41. [PubMed]
- Lutz ST, Chow EL, Hartsell WF, et al. A review of hypofractionated palliative radiotherapy. Cancer 2007;109:1462-70. [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [PubMed]
- Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol 2014;15:164-71. [PubMed]
- Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA 2013;310:1501-2. [PubMed]
- Chow E, Hahn CA, Lutz ST. Global reluctance to practice evidence-based medicine continues in the treatment of uncomplicated painful bone metastases despite level 1 evidence and practice guidelines. Int J Radiat Oncol Biol Phys 2012;83:1-2. [PubMed]
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med 1992;327:614-9. [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [PubMed]
- Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976) 2009;34:69-73. [PubMed]
- Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol 2005;23:3366-75. [PubMed]
- Maranzano E, Trippa F, Casale M, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. [PubMed]
- Rades D, Lange M, Veninga T, et al. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2011;79:524-30. [PubMed]
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer 2013;119:897-903. [PubMed]
- Samant R, Gooi AC. Radiotherapy basics for family physicians. Potent tool for symptom relief. Can Fam Physician 2005;51:1496-501. [PubMed]
- Kirkbride P, Barton R. Palliative radiation therapy. J Palliat Med 1999;2:87-97. [PubMed]
- Smith SC, Koh WJ. Palliative radiation therapy for gynaecological malignancies. Best Pract Res Clin Obstet Gynaecol 2001;15:265-78. [PubMed]
- National Quality Forum: About us. Available online: http://www.qualityforum.org/story/About_Us.aspx, most recently accessed August 20, 2014.
- ASTRO Clinical Practice: All Guidelines. Available online: https://www.astro.org/Clinical-Practice/Guidelines/All-Guidelines.aspx, most recently accessed August 20, 2014.
- Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys 2009;75:1501-10. [PubMed]
- National Quality Forum: NQF endorses cancer measures. Available online: http://www.qualitymeasures.ahrq.gov/content.aspx?id=47285
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [PubMed]
- de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer 2009;17:757-62. [PubMed]


