
Erlotinib enhanced chemoradiotherapy sensitivity via inhibiting DNA damage repair in nasopharyngeal carcinoma CNE2 cells
Introduction
Southern China is one of the high-incidence areas of nasopharyngeal carcinoma (NPC) in the worldwide. Currently, the effective treatment strategy of NPC is radiotherapy, and the 5-year survival rate was 71.7–89.2%, 50.2–75.4%, 53.1–60.3% and 30.1–37.7% for patients at stage I, stage II, stage III and stage IV, respectively (1-5). For mid and late stage’s patients, the total survival rate decreased significantly due to the high local recurrence rate and metastasis rate by radiotherapy alone. Concurrent chemoradiotherapy (CCRT), as routine therapy strategy of NPC (6,7), accompanying with improved therapeutic effect, high-dosage cytotoxic agents usually lead to serious toxic side reaction. Therapy resistance, especially cisplatin (DDP) resistance, is the main cause of disease failure (8). Hence, a new potent systemic management that leads to the clinically desirable to develop treatments with fewer side effects (9) is urgent for this cancer.
Epidermal growth factor receptor (EGFR), an important member of the ErbB family of receptors, its overexpression is a common characteristic of head and neck squamous cell carcinomas (HNSCC), and it usually predicts a poor clinical outcome. By binding to the ligand, EGFR is phosphorylated and activated, which leads to activation of the downstream signaling cascades, facilitating carcinoma cells proliferation, migration and invasion and inhibiting apoptosis. The best-known involved pathways include the rat sarcoma (Ras)/mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) signaling pathways, whose roles in promoting tumor growth, survival, and progression are well characterized (10). Besides, EGFR modulates DNA double-strand break (DSB) repair by regulating non-homologous end-joining via MAPK signaling (11).
Eroltinib is a small, reversible tyrosine kinase inhibitor that competes with adenosine triphosphate for binding to the intracellular catalytic domain of EGFR, inhibiting phosphorylation. In the field of lung cancer and other cancers, there are many reports demonstrate the antitumor efficacy of EGFR inhibitors in combination treatment, especially in the treatment of non-small cell lung cancer and squamous-cell lung cancer (12-14). In a study of an anti-angiogenesis drug combination of erlotinib and a ruthenium-based compound, Berndsen et al. (15) validated the drug combination results in strong synergistic inhibition of cell viability in human endothelial and human ovarian carcinoma cells and also confirmed effective anti-angiogenic and anti-proliferative activity in vivo. Yoo et al. (16) and Gilbert et al. (17) also demonstrated that erlotinib combined with radiotherapy and chemotherapy improved the effective rate of advanced HNSCC. Anisuzzaman and colleagues examined the combined effects of erlotinib and BKM120 on cell growth suppression, apoptosis and signaling pathway, and they demonstrated that cotargeting of EGFR and PI3K is synergistic and induces apoptosis of HNSCC cell lines by inhibiting both axes of the AKT-mTOR pathway and translational regulation of antiapoptotic Bcl-2 proteins (18). But because the biological characteristics of NPC are quite different from the other HNSCCs, it is not clear whether erlotinib enhances the efficacy of chemoradiotherapy on NPC.
The aim of this study was to evaluate the combined effects of erlotinib and chemoradiotherapy on biological characteristics of NPC cells and explore the possible reasons.
Methods
Regents and cell culture
Erlotinib was obtained from Roche (Basel, Switzerland), Dulbecco’s modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) was purchased from Gibco (Glasgow, UK). Rabbit anti-human monoclonal antibodies and horseradish peroxidase (HRP) labeled anti-rabbit secondary antibody were purchased from Cell Signaling Technology Com (Danvers, US). Human poorly differentiated NPC cell line CNE2 (purchased from Cell Bank of Chinese Academy of Science) was cultured in DMEM at 37 °C humidified atmosphere containing 5% CO2. The medium was supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin. Cells were exposed to X-ray of 4 Gy using an X-ray irradiation system.
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay
CNE2 cells in logarithmic growth phase were seeded into 96-well plates (5,000 cells/well), erlotinib (at 0, 10, 20, 40, 80, 160, 320 mmol/L) or DDP (at 0, 0.25, 0.5, 1, 2, 4, 8 mg/L) was added into the medium in advance. Then, cells were continued to be cultured for 24, 48 and 72 h. Following the addition of 20 µL MTT to each well, cells were incubated for 2 h. The absorbance was read at a wavelength of 490 nm. The experiment was performed in triplicate and repeated at least three times. The half maximal inhibitory concentration (IC50) values, IC10 (10% inhibitory concentration values) and IC75 (75% inhibitory concentration values) were calculated using SPSS 16.0 software. Based on the results, we chose the optimal working concentrations to continue the experiments.
Next, cells were divided into control group and four treatment groups (Group I–IV). Control group was set as blank group without any treatment, the four treatment groups all received irradiation of 4 Gy X-ray. Besides, at the optimal working concentration, erlotinib, DDP and erlotinib combined with DDP was given Group II, Group III and Group IV, respectively. Irradiation (dose rate of 1 Gy/min) was followed after 12 h of administration. At 24 and 48 h post-irradiation, cell growth-inhibition rate was calculated as follows:
Growth-inhibition rate (%)=(1 − ODtreatment / ODblank)×100%.
Boyden’s chamber assay and cell scratch test
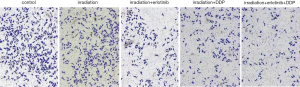
The chambers were put into 24-well plates and coated with Matrigel (100 µL/well) overnight at 37 °C. CNE2 cells were grouped and treated according to MTT assay, and continued to be cultured for 24 h after completion of irradiation. Then they were made into cell suspension (5×105 cells/mL) and seeded into the upper chamber (200 µL/well). Concurrently, the lower chambers were filled with 500 µL medium containing 10% FBS. After 24 h, cells were fixed and stained with crystal violet for 10 min. Cells number was observed microscopically.
For cell scratch test, CNE2 cells in logarithmic growth phase were seeded into 6-well plates (5×105 cells/well) and treated as mentioned above. Cells number of migration was detected using a digital camera system and imaged at 0 and 24 h postirradiation.
Flow cytometry
CNE2 cells seeding in 6-well plates (5×105 cells/well) were treated as mentioned above and fixed in 95% ethanol at 24 h postirradiation. Following rehydration in PBS for 30 min at 4 °C, cells were treated with 1% RNAase for 30 min at 37 °C and stained with propidium iodide for 5 min. Then cells were analyzed using a flow cytometer.
Western blot
Proteins of whole cell extracts were detected by Western blot according to standard protocols. Primary antibodies (anti-EGFR (Cat. # 4267, RRID: AB_2246311, Cell Signaling Technology), anti-ERK1/2 (Cat. # 9102, RRID: AB_330744, Cell Signaling Technology), anti-AKT (Cat. # 9272, RRID: AB_329827, Cell Signaling Technology), anti-p-EGFR (Cat. # 3777, RRID: AB_2096270, Cell Signaling Technology), anti-p-ERK1/2 (Cat. # 9101, RRID: AB_331646, Cell Signaling Technology) and anti-p-AKT (Cat. # 9271, RRID: AB_329825, Cell Signaling Technology)) and HRP labeled anti-rabbit IgG secondary antibody (Cat. # 7074, RRID: AB_2099233, Cell Signaling Technology) were used for determining target proteins levels. Signals were determined via chemiluminescence.
Statistical analysis
SPSS (RRID: SCR_002865) version 16.0 statistical software was used for statistical analysis. Measurement data were presented as means ± standard deviation (SD). Comparisons among multiple groups were analyzed using one-way analysis of variance (ANOVA). Data complying with normal distribution between groups were compared utilizing t-test. Statistical significance was reported if the P value was <0.05.
Results
Erlotinib combined with DDP and irradiation inhibited cell viability
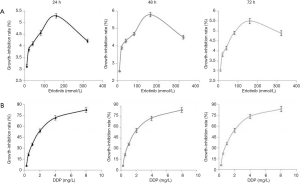
As erlotinib concentrations rose, its inhibitory effect on cell viability enhanced gradually and peaked at 160 mmol/L. However, this inhibitory effect was not dose-dependent because the maximum inhibitory effect was no more than 6% (Figure 1). In contrast, Table 1 showed the inhibitory effect of DDP was dose-dependent, IC10 and IC50 values after 24, 48, 72 h of treatment were 0.264, 0.264, 0.260 mg/L and 1.798, 1.702, 1.691 mg/L, respectively. In order to achieve as low agent cytotoxicity as possible, the optimal working concentration of erlotinib (160 mmol/L) and DDP (1 mg/L) was determined.

Full table
The results of Table 2 and Figure 2 demonstrated erlotinib combined with irradiation could obviously inhibit cell viability, compared with irradiation alone (P<0.05); when cells were treated with erlobinib combined DDP and irradiation, the inhibitory effect was the most significant (P<0.05).

Full table
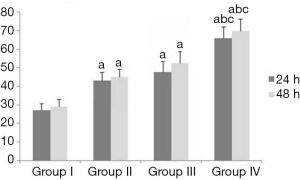
Effects of erlotinib combined with DDP and irradiation on the abilities of invasion and migration, apoptosis and cell cycle distribution of CNE2 cells
After treatment, the results of Table 3, Figure 3 and Figure 4 showed that compared with Group I, the invasion/migration ability of the other three treatment groups all showed significant reduction (P<0.05), and this reduction of Group IV was the most significant (P<0.05). Concerning the apoptosis rate, Group II–IV were all higher than Group I, and Group IV had the highest (P<0.05, Figure 5).
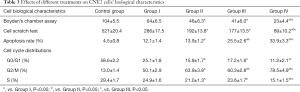
Full table

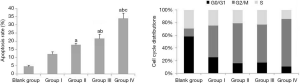
From the perspective of cell cycle distribution, compared with Group I, Group II–IV all showed more obvious cell cycle arrest with blocking to G2/M phase, especially Group IV. These results indicated erlotinib disturbed cell cycle distribution significantly.
The combined effects of erlotinib, DDP and irradiation on EGFR downstream signaling pathways
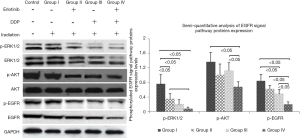
Because EGFR binding to its ligand was strongly related to DNA damage repair of tumor cells after radiotherapy/chemotherapy (11), we speculated erlotinib intervention made it hard for EGFR to bind to ligand, leading to the failure of DNA damage repair. Meanwhile, erlotinib could disturb cell cycle distribution and eventually intensified apoptosis induced by DDP or/and irradiation. Figure 6 showed when erlotinib combined with DDP and irradiation, the reductions of EGFR signal pathways proteins (p-ERK1/2, p-AKT and p-EGFR) expression levels were the most significant (P<0.05), which indicated erlotinib’s inhibitory effect on EGFR signal pathway activation was one of the main mechanisms in combination treatment.
Discussion
Erlotinib can inhibit proliferation, invasion, metastasis, and angiogenesis in tumor cells (19,20). When used as monotherapy, the response rate of erlotinib was not satisfactory. However, the promising disease control rate, time-to-progression and overall survival rate in many trials encouraged researchers to explore the use of erlotinib in conjunction with other anticancer therapies (21-23). In the present study, erlotinib combined with DDP and irradiation showed the most significant reduction in cell viability, invasion and migration ability and the highest increase in apoptosis, when compared to other treatment strategies. These results indicated that erlotinib enhanced the sensitivity of cells to irradiation or/and DDP (24-26).
In order to explore the mechanisms involved, the expression and activation of EGFR downstream signaling pathways proteins in NPC cells were analyzed. Our results demonstrated these proteins expression and activation were inhibited obviously, when cells received erlotinib combined with irradiation and DDP. These results indicated erlotinib prevented activation of EGFR downstream signaling pathways, thus suppressing DNA damage repair in tumor cells (27,28). Though cell cycle G2/M phase arrest was enhanced by combined treatment strategy, this arrest could not be the buffer time for repairing DNA damage induced by irradiation/cytotoxic agent because of erlotinib intervention. Instead, the cell cycle arrest (at G2/M phase) intensified DNA damage induced by DDP or/and irradiation. As a result, erlotinib intensified apoptosis rate, because it suppressed DNA damage repair, tumor cells’ sensitivity to chemoradiotherapy enhanced. Besides, anti-EGFR therapy could reduce the percentage of cells in S phase (because elevated percentage of cells are in G2/M phase, sensitive phase to radiotherapy) (29).
Nevertheless, some studies reported erlotinib monotherapy barely killed tumor cells (30,31). When we designed the optimal working concentration of erlotinib, we found the inhibitory effect on tumor cells growth was very weak (the highest inhibitory effect was less than 6%) and not dose-dependent. This from a side, confirmed erlotinib monotherapy had little effect on CNE2 cells. Zhang et al. also confirmed that treatment of NPC cells with erlotinib alone had no significant effect on tumor cell proliferation (32). Given this, it can be assumed that erlotinib takes effect perhaps depending on DNA damage induced by irradiation or cytotoxic agents.
In conclusion, in combination treatment with radiotherapy and cytotoxic agents, erlotinib enhanced tumor cells’ sensitivity to chemoradiotherapy, whose reason can be attributed to its inhibitory effect on DNA damage repair, and then reducing the chemoresistance and radiotherapy resistance of tumor cells.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-466
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-466). The authors have no conflicts of interest to declare.
Ethical Statement: The authors of this study are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leong YH, Soon YY, Lee KM, et al. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: A meta-analysis. Head Neck 2018;40:622-31. [PubMed]
- Yuan C, Xu XH, Luo SW, et al. Which neoadjuvant chemotherapy regimen should be recommended for patients with advanced nasopharyngeal caicinoma?: A network meta-analysis. Medicine (Baltimore) 2018;97:e11978. [PubMed]
- Bao L, You B, Shi S, et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 2018;37:2873-89. [Crossref] [PubMed]
- Leung TW, Tung SY, Sze WK, et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head Neck 2005;27:555-65. [Crossref] [PubMed]
- Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011;80:661-8. [Crossref] [PubMed]
- Kong M, Lim YJ, Kim Y. Concurrent chemoradiotherapy for loco-regionally advanced nasopharyngeal carcinoma: treatment outcomes and prognostic factors. Asian Pac J Cancer Prev 2018;19:1591-9. [PubMed]
- Wang K, Dong J, He S, et al. Comparison of weekly and triweekly cisplatin regimens during concurrent chemoradiotherapy for nasopharyngeal carcinoma. BMC Cancer 2019;19:482. [Crossref] [PubMed]
- Galluzzi L, Vitale I, Michels J, et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 2014;5:e1257. [Crossref] [PubMed]
- Yeo ELL, Li YQ, Soo KC, et al. Combinatorial strateties of radiotherapy and immunotherapy in nasopharyngeal carcinoma. Chin Clin Oncol 2018;7:15. [Crossref] [PubMed]
- De Luca A, Maiello MR, D’Alessio A, et al. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 2012;16:S17-27. [Crossref] [PubMed]
- Kriegs M, Kasten-Pisula U, Rieckmann T, et al. The epidermal growth factor receptor modulates DNA double-strand break repair by regulating non-homologous end-joining. DNA Repair (Amst) 2010;9:889-97. [Crossref] [PubMed]
- Garon EB, Siegfried JM, Stabile LP, et al. Randomized phase II study of fulvestrant and erlotinib compared with erlotinib alone in patients with advanced or metastatic non-small cell lung cancer. Lung Cancer 2018;123:91-8. [Crossref] [PubMed]
- Tagliamento M, Genova C, Rijavec E, et al. Afatinib and erlotinib in the treatment of squamous-cell lung cancer. Expert Opin Pharmacother 2018;19:2055-62. [Crossref] [PubMed]
- Veschi S, De Lellis L, Florio R, et al. Effects of repurposed drug candidates nitroxoline and nelfinavir as single agents or in combination with erlotinib in pancreatic cancer cells. J Exp Clin Cancer Res 2018;37:236. [Crossref] [PubMed]
- Berndsen RH, Weiss A, Abdul UK, et al. Combination of ruthenium(II)-arene complex (Ru(eta6-p-cymene)Cl2(pta)) (RAPTA-C) and the epidermal growth factor receptor inhibitor erlotinib results in efficient angiostatic and antitumor activity. Sci Rep 2017;7:43005. [Crossref] [PubMed]
- Yoo DS, Kirkpatrick JP, Craciunescu O, et al. Prospective trial of synchronous bevacizumab, erlotinib, and concurrent chemoradiation in locally advanced head and neck cancer. Clin Cancer Res 2012;18:1404-14. [Crossref] [PubMed]
- Gilbert J, Rudek MA, Higgins MJ, et al. A phase I trial of erlotinib and concurrent chemoradiotherapy for stage III and IV (M0) squamous cell carcinoma of the head and neck. Clin Cancer Res 2012;18:1735-42. [Crossref] [PubMed]
- Anisuzzaman AS, Haque A, Wang D, et al. In vitro and in vivo synergistic antitumor activity of the combination of BKM120 and erlotinib in head and neck cancer: mechanism of apoptosis and resistance. Mol Cancer Ther 2017;16:729-38. [Crossref] [PubMed]
- Hirte HW. Profile of erlotinib and its potential in the treatment of advanced ovarian carcinoma. Onco Targets Ther 2013;6:427-35. [Crossref] [PubMed]
- Shao Y, Yu Y, Zong R, et al. Erlotinib has tumor inhibitory effect in human retinoblastoma cells. Biomed Pharmacother 2017;85:479-85. [Crossref] [PubMed]
- Daneshmanesh AH, Hojjat-Farsangi M, Ghaderi A, et al. A receptor tyrosine kinase ROR1 inhibitor (KAN0439834) induced significant apoptosis of pancreatic cells which was enhanced by erlotinib and ibrutinib. PLoS One 2018;13:e0198038. [Crossref] [PubMed]
- Setúbal Destro Rodrigues MF, Gammon L, Rahman MM, et al. Effects of cetuximab and erlotinib on the behavior of cancer stem cells in head and neck squamous cell carcinoma. Oncotarget 2018;9:13488-500. [Crossref] [PubMed]
- Zheng Y, Wang Z, Ding X, et al. Combined erlotinib and PF-03084014 treatment contributes to synthetic lethality in head and neck squamous cell carcinoma. Cell Prolif 2018;51:e12424. [Crossref] [PubMed]
- Nyati MK, Morgan MA, Feng FY, et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer 2006;6:876-85. [Crossref] [PubMed]
- Li XQ, Liu JT, Fan LL, et al. Exosomes derived from gefitinib-treated EGFR-mutant lung cancer cells alter cisplatin sensitivity via up-regulating autophagy. Oncotarget 2016;7:24585-95. [Crossref] [PubMed]
- Yao M, Woods C, Lavertu P, et al. Phase II study of erlotinib and docetaxel with concurrent intensity-modulated radiotherapy in locally advanced head and neck squamous cell carcinoma. Head Neck 2016;38 Suppl 1:E1770-6. [Crossref] [PubMed]
- Keta O, Bulat T, Golić I, et al. The impact of autophagy on cell death modalities in CRL-5876 lung adenocarcinoma cells after their exposure to γ-rays and/or erlotinib. Cell Biol Toxicol 2016;32:83-101. [Crossref] [PubMed]
- Oberthür R, Seemann H, Gehrig J, et al. Simultaneous inhibition of IGF1R and EGFR enhances the efficacy of standard treatment for colorectal cancer by the impairment of DNA repair and the induction of cell death. Cancer Lett 2017;407:93-105. [Crossref] [PubMed]
- Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res 2000;6:2166-74. [PubMed]
- Van Allen EM, Lui VW, Egloff AM, et al. Genomic Correlate of Exceptional Erlotinib Response in Head and Neck Squamous Cell Carcinoma. JAMA Oncol 2015;1:238-44. [Crossref] [PubMed]
- Gibbons DL, Byers LA. A. HER 1-2 punch: dual EGFR targeting deals resistance a deadly blow. Cancer Discov 2014;4:991-4. [Crossref] [PubMed]
- Zhang HH, Yuan TZ, Li J, et al. Erlotinib: An enhancer of radiation therapy in nasopharyngeal carcinoma. Exp Ther Med 2013;6:1062-66. [Crossref] [PubMed]


