
A review of ongoing trials of stereotactic ablative radiotherapy for oligometastatic disease in the context of new consensus definitions
Introduction
The characterization and treatment of oligometastatic disease (OMD) are rapidly evolving areas of research. The term “oligometastases” was originally proposed by Hellman and Weichselbaum in 1995 in reference to an intermediary state of cancer between local disease and widespread metastasis, where the “facility for metastatic growth has not been fully developed and the site for such growth is restricted” (1). It is posited that this state would be amenable to treatment with curative intent using locally targeted techniques such as surgery and radiation. Advances in local treatments, such as radiofrequency ablation and stereotactic ablative body radiotherapy (SABR), have led to an increase in research to determine if treating OMD results in any measurable benefit to patient outcomes. A variety of terms have been used to further describe different states of OMD, such as synchronous, metachronous, and oligoprogression. With each of these terms growing in popularity, the variability between how each is defined has grown as well (2).
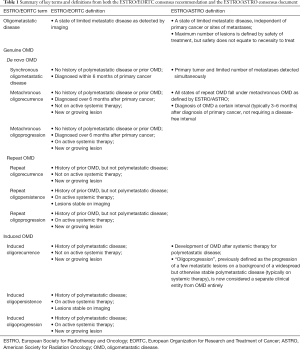
As a result, the European Society for Radiotherapy and Oncology (ESTRO) in conjunction with the European Organization for Research and Treatment of Cancer (EORTC) released consensus recommendations proposing a standardized classification system for OMD (3). In addition to this, the American Society for Radiation Oncology (ASTRO) and ESTRO have also drafted a consensus document to define several terms used in OMD (4). A summary of terms and definitions from both statements is available in Table 1. OMD was defined as a limited number of metastases which could be safely treated with metastasis-directed therapy (MDT). No upper limit was placed on the number of metastases, in part due to a lack of research supporting any maximum number in terms of either safety or efficacy (4). Broadly, states of OMD were categorized into either de novo, repeat or induced (3). Patients with history of prior widespread metastasis were considered induced, patients with prior history of OMD were considered repeat, and patients with neither were de novo. Each of these were further subcategorized into oligorecurrence if patients were off systemic therapy at the time of OMD diagnosis, or oligopersistence and oligoprogression depending on whether lesions progress while patients were on active systemic treatment.
Full table
Our group previously highlighted that significant efforts are already underway to prospectively evaluate SABR in this setting (2). The goal of this study was to build upon our previous work and review ongoing trials evaluating SABR in OMD in the context of new key definitions from both statements.
Methods
A search was completed using the clinicaltrials.gov registry, which includes publicly and privately funded clinical studies worldwide. The search was performed from inception to February 7, 2020 using a combination of terms to capture trials reporting on SABR (“stereotactic”, “stereotaxis”) for oligometastases (“oligo”, “metastasis”, “metastases”, “metastatic”). The full entries for each trial were reviewed by two independent reviewers, with a third available in case of discrepancies.
For trials to be included, their inclusion criteria had to limit the number of metastases throughout the whole body, regardless of primary disease site. In the event of multi-arm studies, at least one had to include SABR. Studies evaluating SABR combined with other local or systemic therapies were also eligible. Only trials actively accruing at the time of the search were included.
Data abstracted from studies selected for analysis included study design, primary disease site, target site(s) of SABR, inclusion criteria, and primary endpoints.
Results
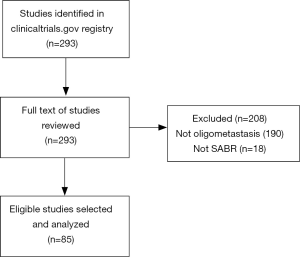
The initial search strategy identified 293 trials, of which 85 met our inclusion criteria after full text review (Figure 1). Of the 208 trials excluded, 190 had populations that were not strictly oligometastatic (i.e., no upper limit on the total number of metastases throughout the body, and therefore potentially polymetastatic), and 18 did not include SABR as an intervention. In particular, many excluded studies had inclusion criteria that identified a maximum number of lesions within one system (e.g., brain or liver), but not throughout the entire body.
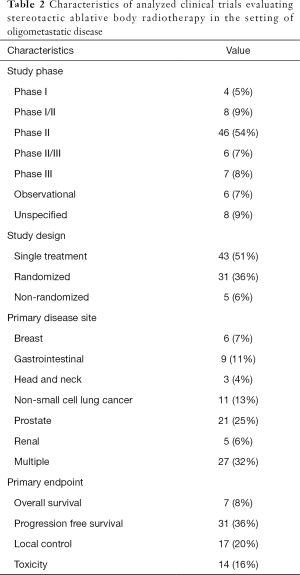
Characteristics of included trials are summarized in Table 2. The majority of trials were phase II (54%, n=46), with the percentage of trials in all other phases being less than 10% each. Of note, a minority were phase III (8%, n=7) and phase II/III (7%, n=6). Over a third of trials were randomized (36%, n=31), with the most common design being a single treatment arm (51%, n=43). Many trials included patients with metastases from multiple primary disease sites (32%, n=27), with the most frequent single disease site being prostate (25%, n=21). Progression-free survival (PFS) was the most common primary endpoint (n=31, 36%), followed by local control (n=17, 20%), toxicity (n=14, 16%) and overall survival (n=7, 8%).
Full table
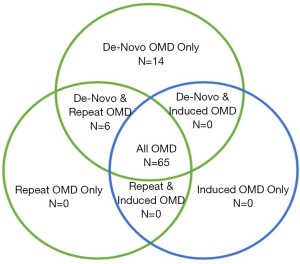
The maximum number of metastatic lesions considered to be OMD varied between one and ten, with the most frequent being five (39%, n=33). A small number of trials (9%, n=8) limited metastases by either size (combined total dimensions or volumes), to a specific location (e.g., adrenal), or feasibility of delivering ablative treatment to a specified location. When evaluating trial population criteria using new consensus classifications, the majority of trials (76%, n=65) included patients from all three broad categories of OMD (Figure 2). A small proportion of trials included patients with de novo disease only (16%, n=14), as well as a combination of de novo and repeat OMD (7%, n=6). No trials investigated solely repeat or induced OMD. Notably, 70 (82%) trials included patients with either metachronous, repeat or induced oligoprogression.
Discussion
In this study, we review 85 currently ongoing trials evaluating the use of SABR for OMD, a year after our initial review of 64 trials using the same eligibility criteria (2). This updated study focuses on assessing these trials in the context of recently released consensus definitions from the ESTRO/EORTC and ESTRO/ASTRO, with an emphasis on evaluating how well trial populations fit within newly defined OMD categories. The characteristics of presently included trials remained largely unchanged, with similar distributions of study phase, study design, primary disease site, and primary endpoint. While this highlights that there are increasing efforts to evaluate the use of SABR in OMD prospectively, most trials remain non-randomized, and include a multitude of primary disease sites.
Whilst there are many retrospective reports and several single-arm prospective studies evaluating the role of MDT for OMD (5,6), a limited number of randomized trials have been published. The first was a 2016 report by Gomez et al. of a multicenter randomized trial of patients with synchronous oligometastatic non-small cell lung cancer (NSCLC). OMD was defined as three or fewer metastases, and patients were randomized to systemic therapy, or to systemic therapy plus MDT in the form of radiation or surgery. The study was terminated early after an interim analysis of 49 patients demonstrated a median progression-free survival (PFS) of 3.9 months in the control group versus 11.9 months in the MDT group [hazard ratio=0.35; 95% confidence interval (CI): 5.72–20.90, P=0.005], with similar rates of toxicities (7). In 2019, further analysis of the same study with increased follow-up, revealed a durable benefit to PFS, with a median of 4.4 months in the control group versus 14.2 months in the treatment (P=0.022) (8). Few other randomized trials have been published since then, including the Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP) trial in 2018, and the SABR versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET) trial in 2019, both of which provided evidence in support of treating OMD (9,10). In STOMP, patients with asymptomatic biochemical recurrence of prostate cancer with 3 or fewer lesions were randomized to either MDT (surgery or radiation) or surveillance with PSA and imaging, with the primary endpoint being androgen deprivation therapy (ADT)-free survival. Sixty-two patients were enrolled, and after a median follow-up of 3 years, ADT-free survival was 13 months in the surveillance group versus 21 months in the MDT group (HR =0.50; 80% CI: 0.40–0.90, P=0.11) (10). Meanwhile, SABR-COMET randomized 99 patients with controlled primaries and 1–5 lesions, in a 1:2 ratio to either palliative standard of care or MDT with SABR, after stratifying by number of metastases (1–3 vs. 4–5). Median overall survival was 28 months in the control group versus 41 months in the MDT group (HR =0.57; 95% CI: 0.30–1.10, P=0.09) (9).
Highlighting the imbalance of the available real-world versus clinical trial data of MDT for OMD, a 2019 review of oligometastatic prostate cancer argued that while MDT may potentially improve various outcomes, only 1 of 14 completed studies were prospective and randomized (11). Additionally, the ESTRO/ASTRO consensus statement on OMD reported that 73 of 97 primary research studies included in their literature review were retrospective (4). Our review demonstrates that several prospective trials are underway, but few are phase III and/or randomized.
A large number of trials were excluded from this review due to the fact that their inclusion criteria, while having an upper limit for lesions within a single organ system, did not place any upper limit on the total number of metastases. As the latest ESTRO/ASTRO consensus document specifies that there is currently no evidence based upper limit for defining OMD, this posed a challenge in identifying trials evaluating true OMD (including oligoprogressive states of OMD) versus polymetastatic disease. Additionally, the ESTRO/EORTC classification system includes 3 distinct classes of oligoprogression, while the current ESTRO/ASTRO consensus states that oligoprogression (i.e., a limited number of progressing lesions on a background of stable widespread metastases, typically while on systemic therapy) should be considered a separate clinical entity than OMD entirely. Returning to Hellman and Weichselbaum’s original paper, OMD was postulated to represent an intermediate state wherein cancers have not yet fully gained the ability to cause widespread metastasis, whereas oligoprogressive cancers have already reached this stage and are in fact progressing in a limited fashion despite systemic therapy.
Indeed, there is evidence suggesting that oligoprogressive disease is a distinct clinical state separate from OMD with worse outcomes (4). For example, a single institutional retrospective analysis showed that among 86 patients with NSCLC treated with SABR to a maximum of four lesions, those with OMD had significantly better PFS than those with oligoprogression (7.6 vs. 3.3 months, P=0.0009) (12). Another single institutional retrospective analysis of 163 patients found that OMD patients had significantly longer median survival compared to those with oligoprogressive disease (34 vs. 22 months, P=0.02) (13).
The majority of trials analyzed in this review had populations spanning all three categories of OMD (de novo, repeat and induced). Some studies have suggested a prognostic difference between different categories of oligometastasis, (i.e., synchronous vs. metachronous) (14). It is likely that de novo synchronous and induced oligoprogressive OMD, at two different ends of the classification spectrum, are very different disease states. As such, further trials could consider differentiating between these entities to better support future clinical decision making. In addition, when evaluating study design, only one third of studies were randomized, which may signal a lack of clinical equipoise or attempts at increasing study recruitment. The overwhelming majority of primary endpoints were non-definitive, including PFS, local control and toxicity. Overall survival (OS) had the strongest support for being able to identify benefit of OMD treatment in the ESTRO/ASTRO consensus (4), however only 8% of trials in this review used OS as a primary endpoint.
The use of SABR for OMD is an active area of prospective research. This review highlights that most currently ongoing trials do not differentiate the newly defined subtypes of OMD. Given this, we would propose consistency of terminology in OMD trials undergoing design to better inform clinical decision making in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Simon Lo, Michael Milano, Tithi Biswas, Charles Simone) for the series “Oligometastasis- Fallacy or Real Deal?” published in Annals of Palliative Medicine. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-847
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-847). The series “Oligometastasis- Fallacy or Real Deal?” was commissioned by the editorial office without any funding or sponsorship. AVL has received honoraria from Varian Medical Systems, AstraZeneca and RefleXion. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Al-Shafa F, Arifin AJ, Rodrigues GB, et al. A review of ongoing trials of stereotactic ablative radiotherapy for oligometastatic cancers: Where will the evidence lead? Front Oncol 2019;9:543. [Crossref] [PubMed]
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. [Crossref] [PubMed]
- Lievens Y, Guckenberger M, Gomez D, et al. Definition of Oligometastatic Disease from a Radiation Oncology perspective: an ESTRO-ASTRO Consensus Document.:1–17. Available from: https://www.astro.org/ASTRO/media/ASTRO/Patient Care and Research/PDFs/OMDManuscriptPC.pdf
- Mazzola R, Jereczek-Fossa BA, Franceschini D, et al. Oligometastasis and local ablation in the era of systemic targeted and immunotherapy. Radiat Oncol 2020;15:92. [Crossref] [PubMed]
- Alongi F, Arcangeli S, Filippi AR, et al. Review and Uses of Stereotactic Body Radiation Therapy for Oligometastases. Oncologist 2012;17:1100-7. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR, Lee JJ, et al. Local Consolidative Therapy versus Maintenance Therapy/ Observation for Patients with Oligometastatic Non-Small Cell Lung Cancer without Progression after Front-Line Systemic Therapy: Results of a Multi-Institutional Phase II Randomized Study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446-53. [Crossref] [PubMed]
- Battaglia A, De Meerleer G, Tosco L, et al. Novel Insights into the Management of Oligometastatic Prostate Cancer: A Comprehensive Review. Eur Urol Oncol 2019;2:174-88. [Crossref] [PubMed]
- Merino Lara T, Helou J, Poon I, et al. Multisite stereotactic body radiotherapy for metastatic non-small-cell lung cancer: Delaying the need to start or change systemic therapy? Lung Cancer 2018;124:219-26. [Crossref] [PubMed]
- Pembroke CA, Fortin B, Kopek N. Comparison of survival and prognostic factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiother Oncol 2018;127:493-500. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]


