Effects of the prolong life with nine turn method (Yan Nian Jiu Zhuan) Qigong on patients with chronic fatigue syndrome: study protocol for a randomized controlled trial
Introduction
Chronic fatigue syndrome (CFS) is a complex chronic medical condition that is characterized by symptom clusters, including pathological fatigue and malaise, that get worse after exertion, cognitive dysfunction, immune dysfunction, unrefreshing sleep, pain, autonomic dysfunction, and neuroendocrine and immune symptoms (1). The International Chronic Fatigue Syndrome Study Group criteria for the illness, often termed the Fukuda criteria, require at least six months of unexplained fatigue, together with the concurrent presence (for at least six months) of at least four of eight symptom criteria (unrefreshing sleep, self-reported impairment in short-term memory or concentration, sore throat, tender cervical or axillary lymph glands, muscle pain, multi-joint pain without joint swelling or redness, headaches of a new type, pattern, or severity, and post-exertional malaise lasting more than 24 h) (2). Depending on the criteria of definition used, CFS affects between 0.8% and 3.4% of the population (3). There is also a high occurrence of fatigue, sleep disturbance, psychiatric comorbidity, and cognitive symptoms (4,5).
Various drugs, such as non-steroidal anti-inflammatory drugs (NSAIDS), antidepressants, and COX-2 inhibitors, have been used to help relieve and manage the symptoms, especially in cases where there is specific medical intervention using highly individualized treatments (6). Additionally, the use of antidepressants is controversial and has significant side effects (7,8). Several reviews have concluded that cognitive behavior therapy (CBT) seems to be a promising treatment for CFS (9,10). However, few people show persistent or sustained significant outcomes in this patient population (11). Many complementary and alternative medicine (CAM) modalities, such as traditional Japanese herbal medicine (Kampo) (12), acupuncture (13), and Qigong (14), have demonstrated to be effective treatment and prevention methods in relieving fatigue, depression, and insomnia.
The practice of Qigong (pronounced “chee gung”) combines breathing, movement, and meditation, and therefore, it is often classified by Western providers under the category of “mind-body medicine” (15). Evidence, to date, supports Qigong’s potential benefits for improving depression, quality of life, and motor function in patients with CFS (16-18).
The prolong life with nine turn method (PLWNT) method is a kind of Qigong that provides overall coordination to help the body achieve a state of relative equilibrium of yin and yang, dredging meridians, and restoring physiological function. It includes eight kinds of massage manipulations on the abdomen and a kind of upper body shaking. Some studies have confirmed the efficacy of massage manipulations for relieving fatigue, sleep quality, and gastrointestinal discomfort (19-21) and to promote beneficial changes in the central nervous system (CNS) (22,23). Other studies suggest that abdominal massage manipulation therapy can cause physiological, neurological, or psychological responses through the b-endorphin release or catabolite elimination to promote a sense of well-being and pain sensation and fatigue relief (21). Functional magnetic imaging (fMRI) as an intuitive and reliable detection technique will be used to provide neuroimaging evidence (24).
To study the clinical effects of PLWNT on treating fatigue, pain, and alleviating depression, this randomized, parallel-group, and single-blinded clinical trial with a sufficient follow-up period was designed. As a preliminary experiment, the aim of this study is to observe the effects of PLWNT treatment on fatigue status using subjective and objective assessments. This trial is ongoing, and the data will be shared through scientific articles. We present the following article in accordance with the SPIRIT Reporting Checklist (available at http://dx.doi.org/10.21037/apm-19-461).
Methods
Design
The study was designed as a randomized, evaluator and statistician blinded, parallel-controlled trial. It will be conducted at the Shanghai University of Traditional Chinese Medicine and the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine. Participants will be allocated to the PLWNT group or the CBT group. The PLWNT group will be treated with Qigong, and the CBT group will be treated with cognitive behavior therapy.
Setting of the study
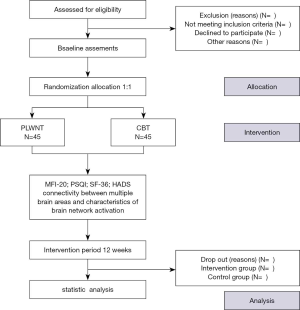
Ninety CFS patients fulfilling the conditions for recruitment of this study will be randomly divided into the PLWNT group and CBT group and distributed according to a 1:1 allocation ratio for a sample size of 40 in each group. However, considering the shedding of clinical cases, it is estimated that this study would require a sample size of 45 in each group, allowing for a 10% dropout rate. The participants in the PLWNT group and CBT group will receive 12 consecutive weeks of treatment and weekly supervised exercise will be conducted at the Shanghai University of Traditional Chinese Medicine taught by a dedicated senior faculty teacher. The number of exercises at home will not be less than six times per week. The total study time required will be 21 weeks: a screening period of one week, a treatment period of 12 weeks, and a follow-up period of 8 weeks. The study flow is depicted in Figure 1.
Randomization and allocation concealment
The eligible participants will be randomly allocated into the PLWNT and CBT groups according to a 1:1 equal proportion rule after a post-baseline assessment. The randomization list will be generated by a statistician using a computer program (Strategic Applications Software, version 9.1.3; SAS Institute Inc., Cary, NC, USA). The statistician, as the producer of the random sequence, numbered the random sequences in order. The sequence will then be placed in a non-transparent envelope by a specified project manager who was not involved in the recruitment, and it will ultimately handed over to the group of researchers. Prior to the implementation of the random assignments, the group of researchers will record details of each participant in the clinical center, including new participants (name, date of birth, and participant and center code, and inclusion date), during reporting and preparation of a signed informed consent. Additionally, after a revision of the participant’s information by the group of researchers to determine if the participant meets the inclusion criteria, a sequence will be randomly selected from the envelope to be labeled on the patient’s data, assigned to each group, and given to the participant.
Informed consent
Prior to the start of the trial, the participants will be briefed on the course of the study and informed about their responsibilities. They will be informed of the physical examinations and precautions. Most importantly, they will be informed that their participation is entirely voluntary, and they can refuse or withdraw at any time, which will not affect their medical or other benefits. Once a participant withdraws, the collected data will not be deleted and will be used for the final analysis. A written informed consent from each participant will be obtained prior to the initiation of any study-related treatment. A research assistant will be responsible for obtaining the informed consent from all participants.
Participants
Participants that meet the following criteria will be included.
- Ages between 20–60 years; no gender requirement.
- Severe chronic fatigue that is unexplained after clinical evaluation and has a history of no less than six months. Fatigue is not caused by the work performed during the trial, and the fatigue is not alleviated after rest.
- At least four of the following eight items: (i) memory or attention drops. Its severity has led to a substantial decline in work capacity, ability to receive education, and ability to engage in social activities and personal life. (ii) Sore throat. (iii) Tender neck or axillary lymph nodes. (iv) Muscle pain. (v) Migratory polyarticular pain without redness and swelling. (vi) Headaches with the type of attack and type and severity different from before. (vii) Pain cannot be alleviated after rest; and (viii) more than 24 hours of muscle pain after exertion.
- Blood, urine routine tests, and liver and kidney functions are normal.
- No participant has received any other treatment plan within one month.
- Participants agree and sign the informed consent.
Participants that meet any of the following criteria will be excluded.
- No fatigue complaints, or less than four symptoms.
- Severe cardiovascular and/or cerebrovascular diseases, endocrine system diseases, sports system diseases, autoimmune diseases, infectious diseases, diabetes, or other mental diseases.
- Taking drugs that may affect the outcome judgment.
- Fatigue symptoms that can be alleviated after rest.
- Fatigue symptoms that do not cause a substantial decline in work ability, educational ability, social activities, recreational activities, and personal life skills.
- A definitive diagnosis of gastrointestinal organic disease, liver and kidney dysfunction, tumor, or other diseases.
- Pregnant or lactating women, drug addiction, heavy metal poisoning, or similar condition.
Participants that meet the following criteria will be excluded after the trial begins.
- The patient has severe physical discomfort or adverse reactions.
- Subject requested withdrawal of informed consent.
- The investigator considers it necessary for the subject to discontinue the study from a medical perspective.
Intervention
PLWNT group
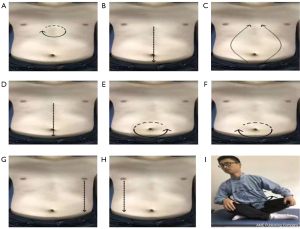
PLWNT includes eight kinds of massage manipulations on the abdomen and an upper body shake, which integrates the techniques of abdominal massage and Qigong guidance. The exercise promotes gastrointestinal motility and secretion and promotes the intestinal nerve chain connection with the advanced nerve center so as to achieve a state in which the body, mind, and spirit are relaxed and harmonious. The nine forms of manipulations can be seen in Figure 2. A Qigong teacher from the Shanghai University of Traditional Chinese Medicine who has been engaged in Qigong education for at least five years will lead the concentrated supervision of the exercise and correct the exercise posture during the entire intervention period for one hour every Sunday. The teacher first leads a five-minute stretching and relaxation exercise that will be performed before the Qigong exercise, and then the teacher will introduce and demonstrate each movement, explain the precautions, and answer questions from participants for five minutes. Subsequently, the teacher will give individual guidance to fifteen participants by correcting their movements for 30 minutes. Finally, all of the participants will be told practice Qigong together for 20 minutes. They will also be told to practice for 30 minutes at home for the remaining six days of the week, practice at WeChat cluster video at 6 o'clock every day, and will do a video supervised practice. If individual participants find it convenient, the private video surveillance exercise will be performed. A “Working Practice Record” will be distributed, and the participants will be required to fill it out after each exercise. The entire treatment process will last for 12 weeks.
CBT group
The experts in relevant fields will be invited for the CBT group to give lectures or psychological counseling on CFS prevention and treatment once a week for one hours. The remaining six days will be taught by a psychologist on the WeChat group video for 30 minutes. Video surveillance participants listen. Lecture videos will be distributed to individual participants who find attending the lectures inconvenient. A “Working Practice Record” will be handed out, and the participants will be required to fill it out after each video study. Treatment will last for 12 weeks. CBT is a psychotherapeutic approach in which elements of behavioral therapy and cognitive therapy approaches are incorporated. In CBT, links are made between the person’s feelings and the patterns of thinking that underpin his or her distress. We will invite qualified CBT therapists that possess appropriate professional qualifications for the provision of CBT [e.g., diploma in CBT or other professionally accredited qualifications involving CBT as a major part of training (e.g., a clinical or counseling psychologist degree)].
Follow-up period
There will be a follow up after 8 weeks from the end of the trial, and all of the participants will return to their original lifestyle. However, all of the participants will be required to record daily exercise or study information. WeChat will be used for the participants to take photos every week to give the researchers the exercise or study they performed every day. At the end of the follow-up period, the scale of fatigue, insomnia, anxiety, and depression will be reassessed. The purpose of the follow-up assessment is to assess the long-term effects of PLWNT on CFS patients. In addition, the participants will be given a special topical pain patch at the end of the follow-up evaluation to increase their follow-up enthusiasm.
fMRI examination procedure
fMRI data will be acquired from all the participants using a 3.0-T Trio Siemens System at the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China. Thirty participants will be scanned: repetition time (TR) =1,900 ms; effective echo times (TE) =2.93 ms; sagittal slices =188; thickness/skip =1.2/0.6 mm; field of view (FOV) =256×256 mm2; matrix =240×256 mm2; Voxel size=1.0×1.0×1.0, phase encoding direction =A > > P and flip angle (FA) =90°. The participants will be requested to rest for ten minutes with their eyes closed, and they will be request to not to think about anything prior to the scan. They will be required to ban head movements during data acquisition. We will acquire 242 three-dimensional image volumes, with the following parameters: TR =2,000 ms; TE =30 ms; section thickness =1 mm; sagittal slices =32; FOV =256×256 mm2; matrix =64×64 mm2; and FA =90°. Both groups of participants will be examined before and after treatment.
fMRI data processing
Imaging data will be processed and analyzed using MATLAB 2015a (MathWorks, Natick, MA, USA), SPM12 (Wellcome Department of Cognitive Neurology, UK), VBM 8 (http://dbm.neuro.uni-jena.de/vbm8/), and CONN toolbox (25). MRICON will be used to convert the original scanned DICOM format to an NIFTI format. The first 10 time points and images will be removed and motion corrected independently to avoid detection of spurious motion artifacts (26). The functional image will be co-registered with the T1 image, and the 10 mm full width half maximum (FWHM) kernel will used to smooth the space to reduce the noise of subsequent image subtraction. The images will detrended, and nuisance variables (including the white matter signal and cerebral spinal fluid signal) will be regressed from the data. The amplitude of low-frequency fluctuations (ALFF) and fractional amplitude of low-frequency fluctuations (fALFF) indices will be computed for each participant.
Functional connectivity (FC) analyses will be performed using the CONN toolbox. Before the correlation analysis, the CompCor algorithm will be used for a linear regression to remove the white matter and ventricular average signals from the data. This step reduces the spatial correlation caused by physiological noise. After Preprocessing (i.e., motion correction, co-registration, subtracting, CBF estimation, smoothing, normalization, and masking), data will be input to the CONN. The seed-to-voxel FC analysis will be performed using a priori ROIs (i.e., superior frontal gyrus (SFG), anterior cingulate cortex (ACC), angular gyrus (AG), hippocampus, pallidum, and postcentral gyrus (PCG). Each voxel of the entire brain is functionally connected to the seed region, and each voxel will have a correlation value. The seed-to-voxel approach analysis generates a correlation graph of Fisher’s r-z transformation for each subject and seeds that subsequently will subjected to independent T-tests.
Outcome measures
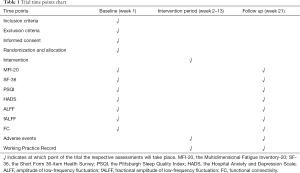
The outcome assessment includes the following items: changes in functional connectivity between multiple brain areas, mental and physical fatigue, anxiety and depression, health status, and sleep quality. The relevant primary outcomes, secondary outcomes, and related self-assessment scale measurement such as the Multidimensional Fatigue Inventory-20 (MFI-20), the Short Form 36-item Health Survey (SF-36), the Pittsburgh Sleep Quality Index (PSQI), and the Hospital Anxiety And Depression Scale (HADS) will be assessed at the baseline and at 13 weeks (at the end of intervention). Changes in the functional connectivity between multiple brain areas, characteristics of brain network activation, and the gastrointestinal microbiome will be tested by the doctors who are experienced in the corresponding departments, but not involved in this trial at the Shanghai Yueyang Hospital of Integrated Traditional Chinese and Western Medicine. The detailed outcome assessment time points are provided in Table 1.
Full table
Primary outcomes
MFI-20
Mental and physical fatigue will be measured using the MFI-20, which is a 20-item self-report instrument designed to measure fatigue. It covers the following dimensions: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity. The MFI-20 consists of 24 statements for which the person has to indicate on a 7-point scale to what extent the particular statement applies to him or her. The statements refer to aspects of fatigue experienced during the previous days. Higher scores indicate a higher degree of fatigue (27).
Secondary outcomes
Changes in functional connectivity between multiple brain areas and characteristics of brain network activation.
Changes in functional connectivity between multiple brain areas including the relationship between the insula, precuneus, thalamus/striatum, cerebellum, occipital and temporal structures, hippocampus, parietal lobule, and characteristics of brain network activation will be explained by temporal changes in functional connectivity between multiple brain areas. Seed to voxel will produce a functional connection between the whole brain and each seed voxel. The correlation between the seed brain region and the BOLD signal of all voxels in the entire brain will be calculated. In addition, ALFF and fALFF analyses will be used to reflect the relevant abnormal brain regions. These changes and characteristics will be assessed by professional radiology technicians at the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine using a Siemens 3.0-T MR scanner with a 32-channel head coil.
SF-36
The health status will be assessed using the SF-36, which includes 36 questions related to an individual’s quality of life that is summarized in two component summary scores: the physical component summary and the mental component summary scores (28). The SF-36 evaluates the following eight physical and mental health areas: physical functioning (PF), physical role functioning (RP), bodily pain (BP), general health (GH), vitality (VT), social role functioning (SF), emotional role functioning (RE), and mental health (MH). Each of the eight areas is scored on a scale of 0–100, where a higher score indicates better health subjectively. These scores are calculated from the questionnaires that were described previously (29).
PSQI
Sleep quality will be measured using the PSQI, which is a self-rated questionnaire that assesses sleep quality and disturbances over a one-month time interval. Nineteen individual items generate seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of the scores for these seven components yields one global score. The total score ranges from 0 to 21, and the higher the score, the poorer the sleep quality (30).
HADS
Anxiety and depression will be assessed using the HADS. The questionnaire comprises seven questions for anxiety and seven questions for depression and takes 2–5 min to complete. Although the anxiety and depression questions are interspersed within the questionnaire, it is vital that these are scored separately (31). In most studies, an optimal balance between sensitivity and specificity is achieved when a case is defined by a score of eight or above on both HADS-A and HADS-D. A sensitivity and specificity for both HADS-A and HADS-D of approximately 0.80 are very similar to the sensitivity and specificity achieved by the General Health Questionnaire. Correlations between HADS and other commonly used questionnaires are typically in the range of 49–83 (32).
Adverse events (AEs) and safety measurements
AEs can be any adverse and non-significant signs (including abnormal experimental findings), symptoms, and diseases associated with the study in time, regardless of drug-related causes (33). Most authors declared that Qigong is a relatively safe approach for various conditions, including hypertension. No severe AEs have been reported. If unexpected AEs occur, defined as any functional lesion caused by the intervention, such as headache, dizziness or vertigo, distension of head, tinnitus, stuffiness in the chest and worsening shortness of breath, heart-pounding or palpitations, muscular soreness or pain, profuse cold perspiration, irritability, neurasthenia, hallucination and paranoia, and psychological stress, regardless of whether it is related to treatment, the nearest affiliated Shuguang Hospital doctor will be notified in time. He/she will then make judgments and start medical treatment.
If serious AEs occur, the investigator will report to the primary investigator and ethics committee to determine whether the participant needs to withdraw from the study and treatment. The investigator will do the utmost to prevent and treat the damage that may result from this study. If, according to the experts’ committee, the adverse event is related to the treatment of the Qigong, the research team will provide the cost of treatment and the corresponding financial compensation for the damage related to the trial.
Sample size calculation
The following two hypotheses are related to the differences between the two groups.
H0:µ1=µ2
H1:µ1≠µ2
where µ1 is the fatigue scale mean score for treating 12 weeks in PLWNT group; and µ2 is the fatigue scale mean score for treating 12 weeks in the CBT group.
According to a previous trial (34), the researchers estimated that the difference in group A was smaller than that in group B. Therefore, in a study with this sample size, the difference between the PLWNT group and the CBT group is considered, and the conservative comparison method Bonferroni is adopted.
In the present study, it is expected that the PLWNT group to be three percentage points better than the CBT group in fatigue scale mean score. The final calculated difference and standard deviation of the two groups of the FSS mean scores was calculated (35,36). Therefore, a sample size of 40 participants should be recruited for each group.
The following formula as used to calculate the sample size in this trial:
(α =0.05, a power of 90%, the two-sided test).
Considering a dropout rate of 10%, each group will require 45 cases. Therefore, a total of 90 participants should be recruited for this randomized controlled trial (RCT).
Statistical analysis
Collected information will be entered into Excel, and the Statistical Package for the Social Sciences (SPSSversion18.0, SPSS Inc., Chicago, IL, USA) will be used to statistically analyze the data. Continuous variables will be summarized using the mean and standard deviation, and the median interquartile range will be used for the non-normal distribution. Categorical variables will be presented by frequency or percentages. The chi-square test will be used to measure the comparability between-group differences for the counting data. An analysis of variance will be applied to the measurement data, such as age and the fatigue scale. For group comparisons of these variables, this study will use a one-way analysis of variance (ANOVA) when the continuous data satisfy a normal distribution or will use the Kruskal–Wallis test when these data do not follow a normal distribution. Normality will be tested using the Kolmogorov Smirnov test. Two-sample independent sample t-tests will be used for the between-group differences, and the effect size will be calculated using Cohen’s d statistics. For within-group comparisons, the paired t-test will be used. Additionally, demographic characteristics between the two groups, baseline measurements, and changes in measurements from baseline to completion of the study will be analyzed using two-sample independent t-tests. If the baseline measurements are different, a covariance analysis (ANCOVA) will be used to control the baseline when comparing the two groups. The clinical overall efficacy evaluation will be performed using a nonparametric test of two independent samples. If the number of volunteers on the subject is too short or there is some missing data, the Mann-Whitney U-test will be used to evaluate the difference between the groups. A P value less than 0.05 indicates that the difference is statistically significant.
Ethics issue
The study protocol is carried out in accordance with the Declaration of Helsinki (as revised in 2013) and the International ethical guidelines for biomedical research involving human subjects (37,38). This study protocol and consent forms were approved by the Ethics Committee of the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai Affiliated Hospital of Shanghai University of Traditional Chinese Medicine (approval number: 81774443) and registered in the Clinical Trial Registry (WHO ICTRP member) on April 12, 2018. The registration number is NCT03496961. The current version of the protocol that has been approved by the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine human research ethics committees is 5.0. The first patient was recruited on December 1, 2018. It is expected that the recruitment (N=90) will be completed by June 2021.
All of the participants will be fully informed about the inclusion and exclusion criteria for this study prior to the start of the trial. They will sign the informed consent form prior to the trial and be informed of the right to withdraw from the trial at any time. Both recruitment and randomization will be open. If our research protocol changes, we must submit a written application to the Research Ethics Committee. Committee members will decide if it is necessary to change the protocol plan. Upon completion of the clinical study, we will submit a study completion report.
Discussion
The impact of chronic fatigue on individuals and society is great, affecting not only the physical and mental health of individuals but also the social development and the economy (39). However, CFS, although highly debilitating, has so far lacked an effective treatment. What is more regrettable is the limited use of evidence-based interventions designed to control symptoms and improve the patient’s functions. Qigong therapy, one of the traditional Chinese wellness practices, is a gentle low-impact mind-body aerobic exercise that primarily emphasizes the combination of the “three regulations”: body focus (posture and movement), breath focus, and mental focus (meditative components) (23). It is simply referred to as optimizing and restoring the body, mind, and spirit (40). Qigong is a relatively safer and more effective treatment for treating CFS compared with other therapy methods (41). It does not require sports space or sports equipment. In recent years, an increasing number of studies have confirmed that Qigong can help to reduce anxiety, improve sleep, and has beneficial effects on the digestive and endocrine systems, both of which contribute to physical and mental health (42,43).
PLWNT is one of the traditional exercises. It was written by a hundred-year-old man named Kai Fang in the Qing Dynasty, and it has a long history. However, no one has ever studied the influence of PLWNT on CFS. According to the self-massage exercise rule, the phrase emphasizing “Insufficient supplementation, shed excess” is of great significance. Other phrases such as “Why borrow medicine to burn Dan, if oneself has the ability to remove disease and prolong life-span?” are found in Yi Shen Ji. PLWNT is composed of eight kinds of massage manipulations on the abdomen and a kind of upper body shaking. It is characterized by static work as an aid, focusing on the manipulation of the abdomen in terms of breathing and thinking regulation, the main use of normal abdominal breathing is to gradually increase the concentration of thinking in the lower Dantian (23). More importantly, during a session, the participants can practice while lying down or sitting, completely free from time and space constraints.
The effect of PLWNT is primarily to strengthen the connection between the brain and the intestine through the Brain-Gut Axis (BGA), which affects the digestive and nervous systems. The connection between the two systems is strengthened primarily through the BGA, which is a complex two-way communication pathway that links the neuroendocrine, intestinal, and immune systems. Abdominal manipulation can stimulate the abdominal and intestinal smooth muscles, enhance gastrointestinal motility, regulate abdominal blood flow and lymphatic system function, and improve the peristaltic function of large and small intestines, effectively preventing digestive diseases. In addition, the pain, emotion, and behavior of the nerve chain linked to the central nervous system and the high-grade nerve center strengthen the connection between the brain nerve and the gastrointestinal digestive system to regulate fatigue, sleep, and mood. In addition, abdominal manipulation can affect the fat in the abdomen, which can help to prevent diseases such as obesity. In previous studies, Qigong improved fatigue, depression, and sleep in patients with CFS (44,45). However, so far, no studies have been conducted to introduce PLWNT Qigong to regulate fatigue, depression, and sleep.
In the evaluation of efficacy, in addition to using the MFI-20, SF-36, PSQI, and HADS to assess the clinical curative effect, fMRI will be also chosen (a Siemens 3.0-T MR scanner) to reveal the brain effective mechanism of PLWNT treatment for CFS, as well as to provide valid and visual evidence.
The structure and function of related brain regions will be measured using fMRI, which is a non-invasive method for examining brain activity and structure (46). There are some accessed findings regarding several brain regions that are believe to be connected with CFS. Studies using arterial spin labeling have shown that individuals with CFS have decreased regional cerebral blood-flow, the bilateral insula seed ROI with the lateralized middle temporal gyrus, superior lateral occipital cortex, angular gyrus play a key role in fatigue regulation (3,47,48). Recent neuroimaging studies have shown a significant decrease in SN functional connectivity to the right posterior insula, which was related to fatigue symptoms (46,49,50). Other studies have demonstrated altered functional connectivity of several regions associated with cognitive, affective, memory, and higher cognitive function in CFS patients including areas involved in memory [left parahippocampal gyrus (PaHcG)], motor (bilateral pallidum), mood (ACC), and higher-order neurocognitive functions (ACC, AG, and SFG) that will lead to CFS generated symptoms (26,51-53). Additionally, the activated brain region is closely related with CFS, in which 50 brain areas have been shown to be activated in the CFS, such as regions in the PaHcG, the posterior superior temporal sulcus, the precuneus, PCG, the insular gyrus, and the hippocampus (54). It has been shown that the massage can activate the ACC, precuneus, and caudate region to treat CFS-related depression and insomnia (55). It is hypothesized that the PLWNT may stimulate central integrated neural signals and cause change in the correlated brain region’s activity to relieve fatigue, depression, and insomnia.
Although the hypothesis of this study is promising, there are several notable limitations. First, the screening of inclusion criteria is based solely on the self-evaluation scales with no specialized mental and psychological testing equipment, resulting in less stringent inclusion of CFS patients. However, the probability of chronic fatigue caused by medical and psychiatric illnesses is reduced by limiting the participants between the ages of 20 and 60. Second, there may be some potential limitations to the protocol. Ideally, each participant should be blind to the procedure, but this is difficult to achieve in non-pharmaceutical trials, so performance bias may be unavoidable. However, we will attempt to ensure that laboratory technicians, data managers, and statisticians are not involved in both the recruitment and processing of the subject’s data. Finally, the results of the study are limited to ≤60-year-old participants, so the results cannot be extended to the elderly.
Despite the above limitations of the study, there are still many advantages. Compared with other treatments, such as medication and psychological counseling, Qigong has distinct advantages for CFS patients. It is easy to learn, it is self-administered, and it is not limited by time and space. In addition, this is the first Qigong study conducted to achieve therapeutic effects by connecting the intestines and brain. More importantly, the use of fMRI technology to objectively demonstrate the efficacy of PLWNT on CFS will be undertaken in this study. Finally, posters and social platforms will be used to recruit individuals at various social levels, which may increase the sample representativeness.
In short, this is a strict, complete, randomization, and adequate concealment RCT study. There has been no published report on the effect of the PLWNT Qigong exercise. If this study demonstrates a significant intervention effect of PLWNT, it will validate a higher quality treatment for patients with chronic fatigue compared with the current treatment options, and it will optimize their guidance.
Acknowledgments
We are grateful to Min Fang for advice related to the analysis, and we thank Yi Zhou for guiding us.
Funding: This trial is funded by the National Natural Science Foundation of China (Grant No. 81774443), the Three-Year Development Plan Project for Traditional Chinese Medicine [Grant No. ZY(2018-2020)-CCCX-2001-05], and the Graduate Innovation Program (Grant No. Y2019080).
Footnote
Reporting Checklist: The authors have completed the SPIRIT Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-19-461
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-461). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol is conducted in accordance with the Declaration of Helsinki (as revised in 2013) and International ethical guidelines for biomedical research involving human subjects. This study protocol and consent forms were approved by the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai Affiliated Hospital of Shanghai University of Traditional Chinese Medicine (approval number: 81774443) and registered in Clinical Trial Registry (WHO ICTRP member). The registration number is NCT03496961. All of the participants will provide informed consent prior to participation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health 2015;30:223-49. [Crossref] [PubMed]
- Roma M, Marden CL, Flaherty MAK, et al. Impaired Health-Related Quality of Life in Adolescent Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Impact of Core Symptoms. Front Pediatr 2019;7:26. [Crossref] [PubMed]
- Boissoneault J, Sevel L, Robinson ME, et al. Functional brain connectivity of remembered fatigue or happiness in healthy adults: Use of arterial spin labeling. J Clin Exp Neuropsychol 2018;40:224-33. [Crossref] [PubMed]
- Shoenfeld Y, Ryabkova VA, Sheibenbogen C, et al. Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clin Immunol 2020;214:108384. [Crossref] [PubMed]
- Bested A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Insights & Advances in Care. Altern Ther Health Med 2018;24:32-3. [PubMed]
- Castro-Marrero J, Saez-Francas N, Santillo D, et al. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: all roads lead to Rome. Br J Pharmacol 2017;174:345-69. [Crossref] [PubMed]
- Kahl KG, Westhoff-Bleck M, Kruger THC. Effects of psychopharmacological treatment with antipsychotic drugs on the vascular system. Vascul Pharmacol 2018;100:20-5. [Crossref] [PubMed]
- Zhang Z, Zhang L, Zhang G, et al. The effect of CBT and its modifications for relapse prevention in major depressive disorder: a systematic review and meta-analysis. BMC Psychiatry 2018;18:50. [PubMed]
- Geraghty KJ, Blease C. Cognitive behavioural therapy in the treatment of chronic fatigue syndrome: A narrative review on efficacy and informed consent. J Health Psychol 2018;23:127-38. [PubMed]
- Strand EB, Nacul L, Mengshoel AM, et al. Myalgic encephalomyelitis/chronic fatigue Syndrome (ME/CFS): Investigating care practices pointed out to disparities in diagnosis and treatment across European Union. PLoS One 2019;14:e0225995. [PubMed]
- Chambers D, Bagnall AM, Hempel S, et al. Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review. J R Soc Med 2006;99:506-20. [PubMed]
- Melby MK, Yoshino T, Tonob D, et al. Differences in demographics and complementary and alternative medicine use between patients attending integrative kampo versus biomedical clinics in Japan. Complement Ther Med 2019;46:202-9. [Crossref] [PubMed]
- Zhang Q, Gong J, Dong H, et al. Acupuncture for chronic fatigue syndrome: a systematic review and meta-analysis. Acupunct Med 2019;37:211-22. [PubMed]
- Chan JS, Li A, Ng SM, et al. Adiponectin Potentially Contributes to the Antidepressive Effects of Baduanjin Qigong Exercise in Women With Chronic Fatigue Syndrome-Like Illness. Cell Transplant 2017;26:493-501. [Crossref] [PubMed]
- Putiri AL, Close JR, Lilly HR, et al. Qigong Exercises for the Management of Type 2 Diabetes Mellitus. Medicines (Basel) 2017;4:59. [Crossref] [PubMed]
- Chan JSM, Ng SM, Yuen LP, et al. Qigong exercise for chronic fatigue syndrome. Int Rev Neurobiol 2019;147:121-53. [Crossref] [PubMed]
- Jiao J, Russell IJ, Wang W, et al. Ba-Duan-Jin alleviates pain and fibromyalgia-related symptoms in patients with fibromyalgia: results of a randomised controlled trial. Clin Exp Rheumatol 2019;37:953-62. [PubMed]
- Zou L, Pan Z, Yeung A, et al. A Review Study on the Beneficial Effects of Baduanjin. J Altern Complement Med 2018;24:324-35. [Crossref] [PubMed]
- Noh GO, Park KS. Effects of aroma self-foot reflexology on peripheral neuropathy, peripheral skin temperature, anxiety, and depression in gynaecologic cancer patients undergoing chemotherapy: A randomised controlled trial. Eur J Oncol Nurs 2019;42:82-9. [Crossref] [PubMed]
- Zengin L, Aylaz R. The effects of sleep hygiene education and reflexology on sleep quality and fatigue in patients receiving chemotherapy. Eur J Cancer Care (Engl) 2019;28:e13020. [Crossref] [PubMed]
- Bender PU, Luz CMD, Feldkircher JM, et al. Massage therapy slightly decreased pain intensity after habitual running, but had no effect on fatigue, mood or physical performance: a randomised trial. J Physiother 2019;65:75-80. [Crossref] [PubMed]
- Lim JH, Kim H, Jeon C, et al. The effects on mental fatigue and the cognitive function of mechanical massage and binaural beats (brain massage) provided by massage chairs. Complement Ther Clin Pract 2018;32:32-8. [Crossref] [PubMed]
- Guo Y, Xu M, Zhang J, et al. The effect of Three-Circle Post Standing (Zhanzhuang) Qigong on the physical and psychological well-being of college students: Study protocol for a randomized controlled trial. Medicine (Baltimore) 2018;97:e12323. [Crossref] [PubMed]
- Wu X, Zhang W, Qin Y, et al. Effect of acupuncture and its influence on cerebral activity in perimenopausal insomniacs: study protocol for a randomized controlled trial. Trials 2017;18:377. [Crossref] [PubMed]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125-41. [Crossref] [PubMed]
- Boissoneault J, Letzen J, Lai S, et al. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: an arterial spin-labeling fMRI study. Magn Reson Imaging 2016;34:603-8. [Crossref] [PubMed]
- Antonio DAF, Muller AG, Butcher R. Reliability and viability of using the Multidimensional Fatigue Inventory-20 in patients with chronic coronary artery disease. Rev Esc Enferm USP 2019;53:e03511. [Crossref] [PubMed]
- Crawley EM, Gaunt DM, Garfield K, et al. Clinical and cost-effectiveness of the Lightning Process in addition to specialist medical care for paediatric chronic fatigue syndrome: randomised controlled trial. Arch Dis Child 2018;103:155-64. [Crossref] [PubMed]
- Suzuki M, Ishikawa T, Sakuma A, et al. Evaluation of the health-related quality of life using the 36-item short form health survey in patients with chronic hepatitis C receiving pegylated interferon/ribavirin/telaprevir triple treatment. Exp Ther Med 2016;12:3353-8. [Crossref] [PubMed]
- Magro R, Camilleri L, Borg AA. Translation and validation of the Fatigue Severity Scale, Pittsburgh Sleep Quality Index and Modified Health Assessment Questionnaire into the Maltese Language, in a cohort of Maltese Systemic Lupus Erythematosus patients. Mediterr J Rheumatol 2017;28:192-200. [Crossref] [PubMed]
- Internet-Delivered Cognitive Behavioural Therapy for Major Depression and Anxiety Disorders. A Health Technology Assessment. Ont Health Technol Assess Ser 2019;19:1-199.
- Wu Y, Fu C, Zhang W, et al. The dermatology life quality index (DLQI) and the hospital anxiety and depression (HADS) in Chinese rosacea patients. Psychol Health Med 2018;23:369-74. [Crossref] [PubMed]
- Aizpuru F. Adverse Events as End Points: The Need to Account for Both Sides of the Same Coin. J Am Heart Assoc 2017;6:e006018. [Crossref] [PubMed]
- Kim JE, Hong KE, Kim HJ, et al. An open-label study of effects of acupuncture on chronic fatigue syndrome and idiopathic chronic fatigue: study protocol for a randomized controlled trial. Trials 2013;14:147. [Crossref] [PubMed]
- Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome: a randomized controlled trial. BMJ 1996;312:22-6. [Crossref] [PubMed]
- Cumming TB, Packer M, Kramer SF, et al. The prevalence of fatigue after stroke: A systematic review and meta-analysis. Int J Stroke 2016;11:968-77. [Crossref] [PubMed]
- Issue Information-Declaration of Helsinki. J Bone Miner Res 2018;33. BM i-BM ii.
- Mathur R, Swaminathan S. National ethical guidelines for biomedical & health research involving human participants, 2017: A commentary. Indian J Med Res 2018;148:279-83. [Crossref] [PubMed]
- Keser İ, Ozdemir K, Erer D, et al. Differences in pain, fatigue, and quality of life in patients with chronic venous insufficiency based on physical activity level. Turk Gogus Kalp Damar Cerrahisi Derg 2020;28:76-83. [Crossref] [PubMed]
- Fulop JA, Grimone A, Victorson D. Restoring Balance for People with Cancer Through Integrative Oncology. Prim Care 2017;44:323-35. [Crossref] [PubMed]
- Guo Y, Xu MM, Huang Y, et al. Safety of Qigong: Protocol for an overview of systematic reviews. Medicine (Baltimore) 2018;97:e13042. [Crossref] [PubMed]
- Gao R, Tao Y, Zhou C, et al. Exercise therapy in patients with constipation: a systematic review and meta-analysis of randomized controlled trials. Scand J Gastroenterol 2019;54:169-77. [Crossref] [PubMed]
- Liu T, Bai S, Zhang RC. Effects of Health Qigong Baduanjin on diabetes related indexes in middle-aged obese women. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2018;34:19-22. [PubMed]
- Yeh ML, Chung YC. A randomized controlled trial of qigong on fatigue and sleep quality for non-Hodgkin's lymphoma patients undergoing chemotherapy. Eur J Oncol Nurs 2016;23:81-6. [Crossref] [PubMed]
- Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson's disease: A systematic review. PLoS One 2017;12:e0181515. [Crossref] [PubMed]
- Josev EK, Malpas CB, Seal ML, et al. Resting-state functional connectivity, cognition, and fatigue in response to cognitive exertion: a novel study in adolescents with chronic fatigue syndrome. Brain Imaging Behav 2019;25:s11682-87. [Crossref] [PubMed]
- Boissoneault J, Letzen J, Lai S, et al. Static and dynamic functional connectivity in patients with chronic fatigue syndrome: use of arterial spin labelling fMRI. Clin Physiol Funct Imaging 2018;38:128-37. [Crossref] [PubMed]
- Staud R, Boissoneault J, Craggs JG, et al. Task Related Cerebral Blood Flow Changes of Patients with Chronic Fatigue Syndrome: An Arterial Spin Labeling Study. Fatigue 2018;6:63-79. [Crossref] [PubMed]
- Wortinger LA, Endestad T, Melinder AM, et al. Aberrant Resting-State Functional Connectivity in the Salience Network of Adolescent Chronic Fatigue Syndrome. PLoS One 2016;11:e0159351. [Crossref] [PubMed]
- Decroix L, van Schuerbeek P, Tonoli C, et al. The effect of acute cocoa flavanol intake on the BOLD response and cognitive function in type 1 diabetes: a randomized, placebo-controlled, double-blinded cross-over pilot study. Psychopharmacology (Berl) 2019;236:3421-8. [Crossref] [PubMed]
- Natelson BH, Vu D, Coplan JD, et al. Elevations of Ventricular Lactate Levels Occur in Both Chronic Fatigue Syndrome and Fibromyalgia. Fatigue 2017;5:15-20. [Crossref] [PubMed]
- Barnden LR, Shan ZY, Staines DR, et al. Hyperintense sensorimotor T1 spin echo MRI is associated with brainstem abnormality in chronic fatigue syndrome. Neuroimage Clin 2018;20:102-9. [Crossref] [PubMed]
- Sevel LS, Boissoneault J, Letzen JE, et al. Structural brain changes versus self-report: machine-learning classification of chronic fatigue syndrome patients. Exp Brain Res 2018;236:2245-53. [Crossref] [PubMed]
- Shan ZY, Finegan K, Bhuta S, et al. Brain function characteristics of chronic fatigue syndrome: A task fMRI study. Neuroimage Clin 2018;19:279-86. [Crossref] [PubMed]
- Liu P, Wang G, Liu Y, et al. Disrupted intrinsic connectivity of the periaqueductal gray in patients with functional dyspepsia: A resting-state fMRI study. Neurogastroenterol Motil 2017;29:e13060. [Crossref] [PubMed]