Primary prophylaxis for venous thromboembolism in ambulatory cancer patients: a systematic review and network meta-analysis
Introduction
Venous thromboembolism (VTE) is a frequent complication encountered in cancer patients which is related to the cancer itself or as a result of surgery and chemotherapy (1,2). The risk of mortality increases as tumor-associated VTE not only leads to fatal thromboembolism events but increases the risk of distant metastases (3-5). Ambulatory patients undergoing chemotherapy often carry a high risk of VTE, which can be high to 30% in those with metastatic or advanced malignancies (6). However, public health efforts have focused on thromboprophylaxis in a short-term setting including hospitalization and major surgery, whilst cancer therapy delivered in the outpatient setting has been ignored (7). For instance, in the updated National Comprehensive Cancer Network (NCCN) Guidelines, all hospitalized patients with active or clinically suspected tumors are recommended to be administered prophylactic anticoagulant therapy throughout their hospital stay without contraindications. However, no recommendations for VTE prevention in ambulatory cancer patients were provided due to their potential risks and limited treatment benefits (8).
Primary prophylaxis for VTE in Ambulatory Cancer Patients have received intense research attention. An array of studies have evaluated the efficacy and safety of anticoagulants in ambulatory patients with cancer and identified that prophylaxis with anticoagulants reduced the risk of VTE by ~50%, with no significant increase in the risk of major bleeding (9). Di Nisio et al. and Akl et al. both found that low molecular weight heparin (LMWH) significantly reduces the incidence of VTE and increased the risk of bleeding (10,11). In addition, with the introduction and widespread use of direct oral anticoagulants (DOACs) in VTE, the prospect of DOACs is rapidly evolving due to the convenience and ease of administration. Some DOACs were found to significantly lower the risk of VTE amongst ambulatory cancer patients with insignificant increases in the risk of bleeding (12). However, comparison of the effectiveness between different classes of anticoagulants are rarely reported, limiting practical recommendations for the prevention of VTE in ambulatory cancer patients despite the range of anticoagulation drugs available. Thus, we conducted a network meta-analysis of randomized studies to compare the effectiveness and safety of current anticoagulant regimens from both direct and indirect evidence in ambulatory patients.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-47).
Methods
A network meta-analyses was used to compare treatments regarding efficacy and safety with direct and indirect evidence of randomized controlled trials (RCTs) (13,14). A frequentist approach with multivariate random effects meta-analysis was used to compare the relative efficacy and safety of candidate strategies to prevent VTE (15-17). Multiple treatments were compared using direct and indirect evidence to provide precise estimates and direct evidence (18). Typical thromboprophylaxis in clinical practice included DOACs, warfarin and LMWH.
Protocols were established in PROSPERO (CRD42019134462) and the network meta-analyses was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for healthcare, by combining systematic reviews and previous network meta-analyses (19). We followed appropriate research approaches defined in the International Society for Pharmacoeconomics and Outcomes Research report on interpreting the comparison between direct and indirect treatments. We referred to the network meta-analysis for healthcare decisions (20).
Data sources and research
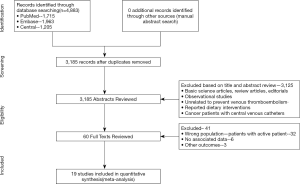
Using controlled vocabulary elements with keywords, we searched PubMed, CENTRAL, and EMBASE electronic databases from inception to the 26th of April 2019 for original reports of RCTs (Figure S1). We collected reference lists of the review articles and identified other suitable trials. Studies were reviewed according to title and abstract to exclude those that did not match the research question. Studies were independently assessed by two reviewers. A third reviewer was used to resolve disputes.
Study selection
Study inclusion criteria: (I) randomized controlled trials (RCTs); (II) adults (aged ≥18 years) ambulatory cancer patients; (III) no obvious thromboembolism; (IV) candidate chemoprevention agents, namely apixaban (5 mg, 10 mg, or 20 mg per day), rivaroxaban, LMWH (prophylactic dosing), semuloparin, aspirin, warfarin alone or combination; (V) a follow-up period of ≥3 months. Exclusion criteria were: (I) hospitalized cancer patients; (II) objectively confirmed venous or arterial thromboembolism at the time of randomization; (III) trials of drugs that are no longer available; (IV) those that did not account for the outcomes of interest.
Data extraction
Data on the primary efficacy outcomes were extracted from studies reporting the composite of objectively confirmed symptomatic or asymptomatic VTE including deep-vein thrombosis and pulmonary embolism diagnosed via the eligibility criteria (computed tomography or routine ultrasonographic testing). Safety outcomes included the rate of major bleeding, clinically relevant non-major bleeding (CRNMB) and all-cause mortality. The occurrence of major bleeding followed the guidelines of the International Society on Thrombosis and Hemostasis (ISTH; bleeding leading to hemoglobin levels ≥2 g per deciliter, transfusion of ≥2 units of packed red blood cells, bleeding that occurs at a critical site (intracranial, intra-spinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitonea, or fatal bleeding) during the intervention period (21). All-cause mortality and clinically relevant non-major bleeding (CRNMB) were defined as overt bleeding that does not meet the criteria for major bleeding but is associated with medical interventions, unscheduled contact with a physician, interruption or discontinuation of study drugs, or discomfort or impairment of activities during daily living (22).
Data synthesis and statistical analysis
The Mantel-Haenszel random-effects model was used to calculate the odds ratio (OR) and 95% confidence intervals (CIs) of the efficacy of the prevention of VTE and the major bleeding events in direct meta-analysis with RevMan v5.3 (23). The I2 statistic was applied to assess statistical heterogeneity, indicating substantial heterogeneity for values over 50% (24). A sensitivity analysis was performed to evaluate the strength of the pooled ORs by exclusion of non-double-blind randomized controlled trials or exclusion of studies with observational periods ≤6 months. When comparing efficacy and safety outcomes, a frequentist framework and random-effects model for Stata v15.1 was employed. The SUCRA ranged from 100 (high likelihood of therapeutic success) to 0 (high likelihood of therapeutic failure) to estimate the probability of each individual treatment related to efficiency and primary safety outcomes.
Results
Characteristics and risk of bias of the included trials
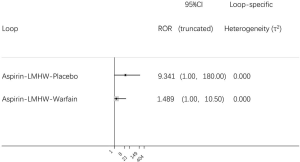
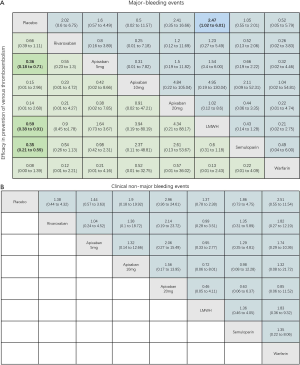
Using the search strategy, 3,185 unique citations were identified and 19 RCTs were included comparing 9 different interventions namely: rivaroxaban, apixaban 5 mg, apixaban 10 mg, apixaban 20 mg, LMWH, semuloparin, aspirin, warfarin and placebo groups (Figure 1) (25-43). A total of 19 trails were performed from 1984 to 2019 consisting of 11,430 patients, of which 897 were exposed to DOACs, 5,673 to traditional anticoagulation therapy and 4,878 placebo controls. All trials were randomized with a follow-up period of 3 months. Sixteen of the trails were multi-center (25-39,42), and 8 were double blind (25,26,28-31,36,37). In 17 of the studies, different candidate agents were compared to placebos in two arm trails. One study compared different apixaban doses to placebos forming a four-arm trial (27). A single study included 3 candidate agents without placebos that were compared in a three-arm trail (43). Table 1 summarizes the characteristics of the included studies and presents the data suitable for the network meta-analysis. Figure 2 shows the direct comparison and network of trials (for primary efficiency outcomes of VTE prevention and safety outcomes of major-bleeding events).
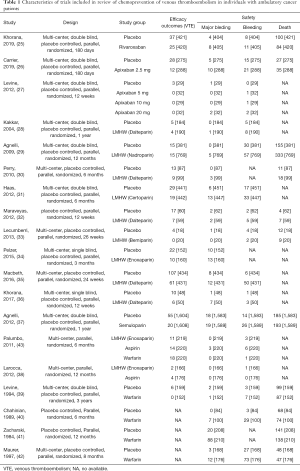
Full table
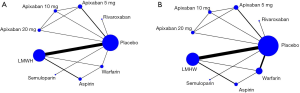
Pairwise meta-analysis
For the primary outcome of efficacy, the results of pairwise meta-analysis showed that apixaban 5 mg (OR 0.36, 95% CI: 0.18–0.71), LMWH (OR 0.50, 95% CI: 0.40–0.64) and semuloparin (OR 0.35, 95% CI: 0.21–0.59) significantly reduced the risk of VTE compared to placebo groups. Other interventions for preventing VTE reduced the risk compared to placebos but the differences lacked significance. Rivaroxaban (OR 0.66, 95% CI: 0.39–1.11), apixaban 10 mg (OR 0.13, 95% CI: 0.01–2.6), apixaban 20 mg (OR 0.12, 95% CI: 0.01–2.36) and warfarin (0.08, 0.00–1.39) were commonly used anticoagulant drugs (Figure S2A).

For major bleeding events, LMWH (OR 1.74, 95% CI: 1.07–2.84) and warfarin (OR 4.66, 95% CI: 1.92–11.31) were significantly related to a higher risk. For the comparison of DOACs with placebo controls, apixaban 5 mg (OR 1.43, 95% CI: 0.36–5.64) and rivaroxaban (OR 2.02, 95% CI: 0.60–6.57) increased the risk of bleeding but the effects were not significant (Figure S2B). Warfarin (OR 3.66, 95% CI: 2.34–5.7) significantly increased the risk of CRNMB, but DOACs (OR 1.48, 95% CI: 0.87–2.51) did not significantly increase the risk. There were no significant differences in all-cause mortality between LMWH (OR 1.14, 95% CI: 0.8–1.62), warfarin (OR 0.85, 95% CI: 0.67–1.08) and DOACs (OR 0.96, 95% CI: 0.62–1.49) compared to placebo controls (Figure S2C,D).
Network meta-analysis and efficacy outcomes
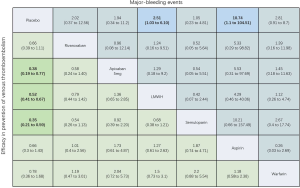
As shown in Figures 3 & 4 (the data of Figure 4 are based on Figure S3), anticoagulant prophylaxis in ambulatory cancer patients could effectively reduce the incidence of VTE compared to placebo groups. The anti-thrombosis effects of apixaban improved regardless of dose. However, only apixaban (5 mg) showed a significant effect (OR 0.36, 95% CI: 0.18–0.71; SUCRA=69.5). Apixaban at 10 mg (OR 0.15, 95% CI: 0.01–2.96; SUCRA=77.9) or 20 mg (OR 0.14, 95% CI: 0.01–2.68; SUCRA=78.2) had modest but non-significant effects on the rate of VTE occurrence. Similarly, rivaroxaban (OR 0.66, 95% CI: 0.39–1.11) and warfarin (OR 0.75, 95% CI: 0.35–1.59) non-significantly reduced the risk of VTE. Notably, both LMWH (OR 0.5, 95% CI: 0.39–0.63; SUCRA=52.1) and semuloparin (OR 0.35, 95% CI: 0.21–0.59; SUCRA=71.4) significantly prevented VTE.

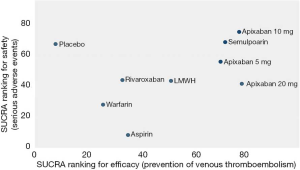
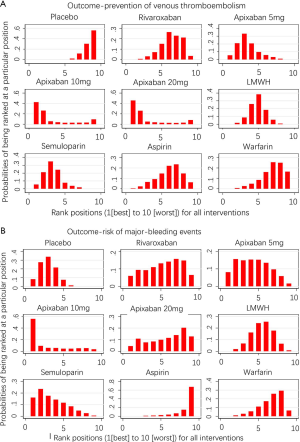
Safety outcomes
Compared to placebo controls, the lowest safety ranking was observed for aspirin (OR 9.65, 95% CI: 1.1–84.36; SUCRA=9.4), followed by warfarin (OR 3.06, 95% CI: 1.03–9.08; SUCRA=29.1), apixaban at 20 mg (OR 2.43, 95% CI: 0.27–21.78; SUCRA=40.4), rivaroxaban (OR 2.02, 95% CI: 0.39–10.41; SUCRA=41.8), LMWH (OR 1.96, 95% CI: 0.99–3.86; SUCRA=44.1), and apixaban at 5 mg (OR 1.41, 95% CI: 0.33–5.93; SUCRA=58.5) (Figure 3).
LMWH (OR 2.25, 95% CI: 1.13–4.47) and warfarin (OR 3.49, 95% CI: 1.33–9.15) significantly increased the risk of CRNMB compared to placebo controls. As the dose of apixaban increased, the risk of CRNMB increased: 5 mg (OR 1.54, 95% CI: 0.36–6.63), 10 mg (OR 2.03, 95% CI: 0.13–3.1), and 20 mg (OR 3.17, 95% CI: 0.25–39.66). For DOACs agents, rivaroxaban (OR 1.38, 95% CI: 0.24–7.99) and apixaban (5 mg) had comparable effects (Figure S4A). No significant differences between placebo and experimental groups in terms of patient deaths were observed (Figure S4B).
Sensitivity analysis
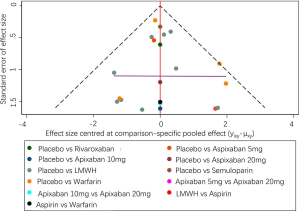
Sensitivity analyses are shown in Figures S4 & S5. The data were comparable to the primary outcome data. The criteria for sensitivity analysis were: (I) the exclusion of non-double-blind randomized controlled trials; (II) the exclusion of studies with observation times ≤6 months. Under the first criteria, for the prevention of VTE in each group, apixaban 5 mg (OR 0.36, 95% CI: 0.18–0.71) and LMWH (OR 0.59, 95% CI: 0.38–0.91) were significantly superior. For safety outcomes, LMWH (OR 2.47, 95% CI: 1.02–6.01) significantly increased the risk of major-bleeding events (Figure S5A). All agents increased the risk of CRNMB compared to placebo controls without significant effects (Figure S5B). When applying the second rule, apixaban 5 mg (OR 0.38, 95% CI: 0.19–0.77) and LMWH (OR 0.52, 95% CI: 0.41–0.67) influenced the efficacy. LMWH (OR 2.51, 95% CI: 1.03–6.1) and increased the risk of major-bleeding events (Figure S6).
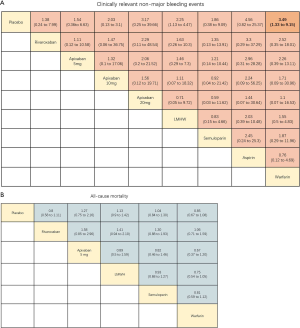
Quality of the evidence and risk of bias
In general, there were no serious risks of bias or inconsistency in the included studies (Figures S7,S8). Following several comparisons, the 95% CI was cross-unified, leading to data inaccuracies. Using direct and independent evidence, we were confident that apixaban at 5 mg (OR 0.36, 95% CI: 0.18–0.71) and LMWH (OR 0.5, 95% CI: 0.39–0.63) prevented VTE in comparison to placebo controls.
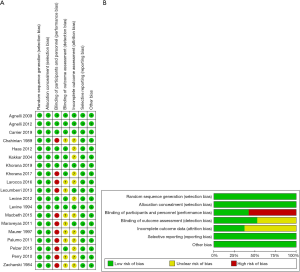
The risk of bias of each included study was evaluated using the Cochrane Collaboration. Random sequence generation and allocation concealment were used to estimate selection bias, participant blinding, personnel to performance bias, blinding of the outcome for detection bias, incomplete outcome data to attrition bias, selective reporting to reporting and other forms of bias (23). The included studies had low bias overall, suggesting the quality of the included trials was high (Figure 5).
Discussion
In this network meta-analysis and systematic review, nine protocols for the primary prevention of VTE in ambulatory cancer patients were assessed. The effectiveness and safety of the various regimens were compared regarding: (I) the efficacy of VTE prevention; (II) major-bleeding events; (III) CRNMB; and (IV) all-cause mortality. According to our analysis, anticoagulant prophylaxis effectively reduced the incidence of VTE and did not significantly increase all-cause mortality. Compared to warfarin and LMWH, apixaban had significant effects on the reduction of thrombosis without increasing the risk of major-bleeding events.
Previous studies compared anticoagulation regimens for active cancer patients (44,45). The probability of developing VTE in ambulatory cancer patients is nearly 5-fold higher than non-tumor patients and the risk of recurrent VTE is 2–9 fold higher (46,47). As the survival time of cancer patients gradually rises, the risk of developing VTE increases (48). In our analysis, anticoagulant prophylaxis in ambulatory cancer patients effectively reduced the incidence of VTE, thereby improving the quality of life of the patients. Compared to warfarin, apixaban effectively prevented VTE in a manner comparable to LMWH. Apixaban at 5 and 20 mg doses and LMWH increased the risk of major-bleeding events whilst the risk of major-bleeding decreased in those receiving apixaban at 10 mg per day. Considering heterogeneity between the outcomes, disparities in the study population and the accuracy of follow-up may explain these discrepancies. Thus, apixaban at the appropriate dose may decrease the risk of VTE without increasing the bleeding risk, but this requires validation in larger study cohorts. Semuloparin significantly prevented VTE (OR 0.35, 95% CI: 0.21–0.59) and increased the risk of major-bleeding events (OR 1.05, 95% CI: 0.29–3.81), but this drug is not commonly available in the clinical and belongs to the group of LMWHs. It is therefore not recommended for future use.
The compliance with medication is vital for ambulatory cancer patients. Anticoagulant prophylaxis with LMWH or warfarin requires frequent blood tests that increase the cost of therapy, enhance patient discomfort, and reduce thromboprophylaxis compliance. Thus, the use of DOACs to prevent VTE are recommend by doctors in the clinic (49). In this meta-analysis, we did not include all DOACs approved by the US Food and Drug Administration including dabigatran etexilate and edoxaban tosylate. The comparison of the effects of each group to prevent VTE requires further assessment. However, we observed good efficacy and high safety of the DOACs which may suggest better compliance. Although the Apixaban (5 mg) group showed the highest prevention of VTE and the lowest risk of major bleeding events, the recommended dosage cannot be administered based on the group sizes. Aspirin is used as an antiplatelet agent in clinical practice and is frequently compared to LMWH in RCTs. Aspirin prevents VTE, but the risk of major bleeding was the highest amongst all included agents. However, some have suggested that the use of aspirin to prevent VTE lowers the risk of bleeding (50). This article included two articles related to aspirin and no direct comparison to placebo groups were performed. To assess the efficacy and safety of aspirin to prevent VTE, further studies are now required.
Our study had some limitations. The meta-analysis was dependent on the quality of the included studies. Although sensitivity analysis did reveal significant bias, some outcome data were absent. Some studies included screening based DVT whilst others did not, which may increase heterogeneity. Because the time factor in the included studies was also insufficient, only the therapeutic effects and safety of the various drugs were compared.
The study also has several strengths. First, we performed a thorough literature search to provide an exhaustive analysis of the available evidence. Secondly, our goal was to provide an OR and the effectiveness and safety of treatments to support clinical decision making. This is the only article to compare the therapeutic effects of various anticoagulant drugs in ambulatory cancer patients. Finally, we provided assessments of direct and network comparisons to consider direct and indirect evidence. This helps describe comparative data from previous systematic reviews in this area.
Conclusions
Based on the analysis of existing clinical RCTs, we identified a range of compounds that can prevent VTE in ambulatory cancer patients. When a risk assessment for thrombosis in ambulatory cancer patients is performed and patients are deemed an intermediate-to-high risk, we recommend anticoagulant prophylaxis. Amongst the commonly used anticoagulant drugs, apixaban improved the thromboprophylaxis effects and lowered the risk of major-bleeding events. Apixaban also does not require the detection of blood indicators, thereby reducing patient discomfort. We thus recommend that apixaban at the appropriate dose may decrease the risk of VTE without increasing the bleeding risk. This now requires validation in larger study cohorts.
Acknowledgments
Funding: This work was supported by a grant from the National Natural Science Foundation of China (No. 81772516).
Footnote
Reporting Checklist: The authors have completed the PRISMA Reporting Checklist. Available at http://dx.doi.org/10.21037/apm-20-47
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-47). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lunenfeld B, Stratton P. The clinical consequences of an ageing world and preventive strategies. Best Pract Res Clin Obstet Gynaecol 2013;27:643-59. [Crossref] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459-544. [Crossref] [PubMed]
- Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-50. [Crossref] [PubMed]
- Blom JW, Vanderschoot JP, Oostindier MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006;4:529-35. [Crossref] [PubMed]
- Otten HM, Mathijssen J, ten Cate H, et al. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: an underestimated phenomenon. Arch Intern Med 2004;164:190-4. [Crossref] [PubMed]
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol 2009;27:4839-47. [Crossref] [PubMed]
- Office of the Surgeon General, National Heart L, Blood I. Publications and Reports of the Surgeon General. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville (MD): Office of the Surgeon General (US); 2008.
- Streiff MB, Holmstrom B, Angelini D, et al. NCCN Guidelines Insights: Cancer-Associated Venous Thromboembolic Disease, Version 2.2018. J Natl Compr Canc Netw 2018;16:1289-303. [Crossref] [PubMed]
- Di Nisio M, Porreca E, Candeloro M, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2016;12:CD008500. [Crossref] [PubMed]
- Di Nisio M, Porreca E, Ferrante N, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2016;12:CD008500. [Crossref] [PubMed]
- Akl EA, Kahale LA, Hakoum MB, et al. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev 2017;9:CD006652. [Crossref] [PubMed]
- Mosarla RC, Vaduganathan M, Qamar A, et al. Anticoagulation Strategies in Patients With Cancer: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:1336-49. [Crossref] [PubMed]
- Mills EJ, Ioannidis JP, Thorlund K, et al. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA 2012;308:1246-53. [Crossref] [PubMed]
- Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159:130-7. [Crossref] [PubMed]
- Bangalore S, Toklu B, Kotwal A, et al. Anticoagulant therapy during primary percutaneous coronary intervention for acute myocardial infarction: a meta-analysis of randomized trials in the era of stents and P2Y12 inhibitors. BMJ 2014;349:g6419. [Crossref] [PubMed]
- Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98-110. [Crossref] [PubMed]
- White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111-25. [Crossref] [PubMed]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. [Crossref] [PubMed]
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. [Crossref] [PubMed]
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011;14:429-37. [Crossref] [PubMed]
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4. [Crossref] [PubMed]
- Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119-26. [Crossref] [PubMed]
- Higgins JPT, Thomas J, Chandler J, et al. editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available online: www.training.cochrane.org/handbook
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med 2019;380:720-8. [Crossref] [PubMed]
- Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med 2019;380:711-9. [Crossref] [PubMed]
- Levine MN, Gu C, Liebman HA, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost 2012;10:807-14. [Crossref] [PubMed]
- Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol 2004;22:1944-8. [Crossref] [PubMed]
- Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol 2009;10:943-9. [Crossref] [PubMed]
- Perry JR, Julian JA, Laperriere NJ, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost 2010;8:1959-65. [Crossref] [PubMed]
- Haas SK, Freund M, Heigener D, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost 2012;18:159-65. [Crossref] [PubMed]
- Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012;48:1283-92. [Crossref] [PubMed]
- Lecumberri R, Lopez Vivanco G, Font A, et al. Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL study. Thromb Res 2013;132:666-70. [Crossref] [PubMed]
- Pelzer U, Opitz B, Deutschinoff G, et al. Efficacy of Prophylactic Low-Molecular Weight Heparin for Ambulatory Patients With Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J Clin Oncol 2015;33:2028-34. [Crossref] [PubMed]
- Macbeth F, Noble S, Evans J, et al. Randomized Phase III Trial of Standard Therapy Plus Low Molecular Weight Heparin in Patients With Lung Cancer: FRAGMATIC Trial. J Clin Oncol 2016;34:488-94. [PubMed]
- Khorana AA, Francis CW, Kuderer NM, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thromb Res 2017;151:89-95. [PubMed]
- Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601-9. [Crossref] [PubMed]
- Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119:933-9. [Crossref] [PubMed]
- Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet 1994;343:886-9. [Crossref] [PubMed]
- Chahinian AP, Propert KJ, Ware JH, et al. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B. J Clin Oncol 1989;7:993-1002. [Crossref] [PubMed]
- Zacharski LR, Henderson WG, Rickles FR, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer 1984;53:2046-52. [Crossref] [PubMed]
- Maurer LH, Herndon JE 2nd, Hollis DR, et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: a Cancer and Leukemia Group B study. J Clin Oncol 1997;15:3378-87. [Crossref] [PubMed]
- Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol 2011;29:986-93. [Crossref] [PubMed]
- Rossel A, Robert-Ebadi H, Combescure C, et al. Anticoagulant therapy for acute venous thrombo-embolism in cancer patients: A systematic review and network meta-analysis. PLoS One 2019;14:e0213940. [Crossref] [PubMed]
- Farge D, Bounameaux H, Brenner B, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2016;17:e452-66. [Crossref] [PubMed]
- Cronin-Fenton DP, Sondergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer 2010;103:947-53. [Crossref] [PubMed]
- Chee CE, Ashrani AA, Marks RS, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood 2014;123:3972-8. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Verdecchia P, Angeli F, Aita A, et al. Why switch from warfarin to NOACs? Intern Emerg Med 2016;11:289-93. [Crossref] [PubMed]
- Wu H, Chen X, Ji J, et al. Progress of Exosomes in the Diagnosis and Treatment of Pancreatic Cancer. Genet Test Mol Biomarkers 2019;23:215-22. [Crossref] [PubMed]