
Legal assistance in dying for people with brain tumors
Introduction
In 1997, Oregon became the first state to allow legal medical assistance in dying, termed under their law “Death With Dignity”, and in 2009 Washington state followed with its own law (1-3). Both states require mandatory reporting of details of access and compile this data for reports accessible to the public (1-3). Several other states and countries have different laws allowing medical assistance in dying, some with varying details (see Table 1). In general, most of these laws require a terminal diagnosis with limited prognosis, and assessment of capacity and competence to make such a decision. Patients who meet these criteria then can be prescribed a combination of medications that, when ingested, will end their life. In Washington and other states who survey physician-perceived reasons for requesting DWD, loss of autonomy, inability to engage in enjoyable activities, loss of dignity and bodily functions, and concerns about being a burden on family and friends are listed as the most common reasons for patients to request access to DWD (1-3). Patients with brain tumors may be more likely to experience such symptoms and concerns earlier and more frequently than patients with other cancers or non-neurologic diseases, making their decisions parallel to patients with non-cancer neurologic diagnoses such as amyotrophic lateral sclerosis (4-6). In Oregon in 2018, approximately 10% of the cancer patients who requested DWD were diagnosed with brain cancer (1). Notably, most reports of patients with systemic cancers requesting DWD do not explicitly collect data on whether metastatic brain tumors were present at the time of the request, although brain metastases are very common in advanced cancer (5). Symptom burdens, especially motor deficits, cognitive changes and dysphagia, are similar in prevalence in patients with CNS lymphoma and brain metastases compared to patients with gliomas, so symptomatic concerns are likely similar across these diseases (5). These common symptoms in patients with CNS tumors might make them more likely to request DWD for reasons of loss of independence and autonomy, while at the same time making decisions on whether they qualify for the legal application of DWD more complex, due to impaired cognitive abilities. Dysphagia is present in 30% of glioma patients at some point in their illness (6), and this certainly should be a consideration in terms of ability to self-administer oral regimens. The literature is lacking in specific data on how common these symptoms are in brain tumor patients seeking DWD, whether they are barriers to access, and whether exceptions to some requirements are appropriate.
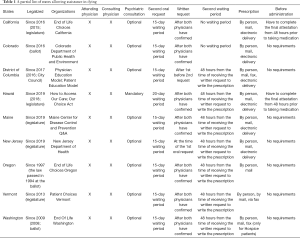
Full table
Decisional capacity in patients with brain tumors
A significant portion of patients with brain tumors lack formal competence or capacity even prior to initial surgery (7,8). Using the MacArthur Competence Assessment Tool for Treatment, 25% of patients were found to lack competence to consent for surgical intervention, and interestingly, male patients and those with higher grade tumors were more likely to lack competence (7,8). The semantic verbal fluency subset (asking patients to name at least ten animals in less than a minute) of the revised Addenbrooks Cognitive Examination was the most predictive single screening tool that predicted cognitive impairment and lack of capacity (7,8). It should be noted that while failing to name ten animals in one minute was very sensitive to detecting incapacity, it was not at all specific, so passing this test did not adequately detect some patients who more detailed testing classified as lacking capacity (7,8). In a large cohort of high grade glioma patients from the Netherlands, their relatives and physicians responded on questionnaires that as many as half of patients were felt to lack capacity from the time of their initial diagnosis but decisions regarding withdrawal of care were ultimately made in the majority of patients by providers and relatives (9).
Decisions regarding capacity for participation in clinical trials is somewhat more complex and has been assessed in patients with malignant gliomas (10). Using the Capacity to Consent to Research Instrument, one-third of patients had significant impairment in reasoning, appreciation and understanding domains, but notably not in simple yes or no choice making (10). Interestingly, education and depression did not correlate with impairment, but lower KPS, anticonvulsants and higher steroid dose each increased the likelihood of impairment. Similar findings have been reported for patients with brain metastases (11).
The legal process for DWD in several states and countries suggest a formal competence evaluation by a psychiatrist and screening for concurrent psychiatric diagnoses, but there remains some debate in the psychiatric literature on whether doing so is ethical, with a portion of psychiatrists surveyed responding that they did not believe it was ethical for them to perform competence evaluations for the purpose of assistance in dying even in patients they believed to be competent (12,13). The American Academy of Neurology Ethics, Law and Humanities Committee in 2018 formally retired a prior position paper opposing the participation of its members in “physician-assisted suicide” or “euthanasia” (14). There continues to be debate within oncology professional societies on the practice, despite implementation at several major cancer centers (15-17). More evidence-based data from patients, caregivers and providers about their experience with these programs before, during and after (especially in patients who obtain access but do not ultimately proceed with administration), could help inform these ongoing debates. In addition, more detailed data is needed on how the common symptoms of brain tumors described above (cognitive impairment, dysphagia, etc.) create obstacles to accessing DWD for these patients.
Medical assistance in dying: a Canadian psychiatrist’s perspective
In 2016, Canada joined a number of other jurisdictions by creating laws permitting assisted death, known in Canada as medical assistance in dying (MAID) (18). To be eligible, patients must reside in Canada, be 18 years or older, and have a grievous and irremediable condition (18). More of a legal term than a medical one, ‘grievous and irremediable’ is defined as a serious and incurable illness causing an irreversible decline in functioning, intolerable physical or psychological suffering, and reasonably foreseeable natural death (RFND). Among other safeguards mandated by law, MAID requesters must undergo two assessments to confirm eligibility, and they must demonstrate decisional capacity to choose MAID both at the time of the assessment as well as on the day that MAID is administered (18).
While Canadian law does not explicitly prohibit patients with psychiatric illness from accessing MAID, most—or perhaps all—patients requesting MAID solely for psychiatric illness will not meet the RFND requirement, and will thus be ineligible (19). It is important to note, however, that individuals with psychiatric illness who otherwise meet all eligibility criteria are indeed eligible for MAID. As a result, MAID requests may come from patients with comorbid physical and psychiatric illness, and, while not required by law, psychiatrists are sometimes asked to assess MAID eligibility for such patients.
The most technically challenging aspect of the assessment is assessing capacity, particularly given that there is no universally accepted approach to assessing capacity for MAID (20). Clinician assessors must assess capacity similarly to other medical interventions, which vary jurisdictionally, but which can generally be categorized into 4 pillars described in a seminal paper by Applebaum (21): Patients must understand the relevant medical information; they must engage in a rational process of manipulating the information; they must appreciate the situation, the condition and its consequences; and they must demonstrate a clear and consistent choice. In essence, the goal is to ensure that patients make an informed decision, and one that is in line with their longstanding values and worldview, rather than a fleeting expression of reversible duress or a delusionally hopeless view of the future.
Eligible MAID requesters are often highly educated, independent, and high functioning individuals who find the prospect of losing their independence and functioning intolerable, and who loathe the thought of being cared for by others as they approach end of life (22). Choosing MAID is often a way of exerting control over their death the way they have exerted control over their lives. Even among MAID requesters with RFND and comorbid psychiatric illness, in the authors experience, we have rarely found psychiatric symptoms to be of such magnitude as to preclude patients’ decisional capacity.
Patients with neurologic disease represent a particularly challenging cohort with respect to MAID. As the MAID law is currently written, patients must demonstrate capacity at the time of assessment as well as immediately prior to assessment. One challenge this presents in patients with CNS disease is that their ability to demonstrate decisional capacity may be precluded by aphasia or other neurologic or cognitive effects of the tumor. In our experiences, many such patients have sought MAID earlier in their illness trajectory than if the law allowed for an advanced directive to choose MAID. Patients have found it stressful having to monitor their symptoms for signs of potential disease progression and living with the fear that they could abruptly lose capacity (and thus eligibility for MAID).
In certain instances, if patients are limited to answering ‘yes or no’ questions, assessors may choose to use a modified version of the Western Aphasia Battery (23) subscale on auditory comprehension—patients must answer a series of ‘yes or no’ questions—whereby a high degree of correct answers may support an assumption of patients’ comprehension and expressive abilities. Researchers have studied whether other screening tools or even certain clinical variables can predict capacity (or lack of capacity) among patients with brain tumors (7). Ultimately, no tool is fully accurate, and any number of false positives or negative may be considered too a high a risk for a medical decision as important as MAID. As such, predictive tools cannot be relied upon as a valid proxy for a full in-depth assessment. In acknowledging the challenges posed to patients with CNS tumours who request MAID, we often recommend starting the MAID assessment process early, so that assessors can learn about the patient, their values, and the consistency of their decisions at multiple time points. In doing so, assessors can be more confident that patients’ expressed wishes are part of their longstanding and core values, rather than an error or misunderstanding of the patient due to the communication barriers that are often seen in patients with brain tumors.
Currently, the RFND criterion is being challenged in Canadian courts, which may conceivably lead to an expansion of MAID eligibility to include patients who are not approaching the natural end of life (24). In that case, MAID could conceivably become permissible for patients with severe, treatment refractory psychiatric illness (without RFND) who are suffering intolerably, akin to the current practice in the Netherlands and Belgium (20). There, stakeholder and governmental agencies have made a number of recommendations when assessing eligibility of patients solely with psychiatric illness. Among them, guidelines suggest: extending the mandatory reflection period to one year; attempting to treat the underlying psychiatric illness before undergoing assisted death; involving family members or other key caregivers; and consulting current or past treatment providers as part of the assessment process (20). Those who oppose expanding MAID eligibility have argued that mental illness inherently precludes patients’ agency, autonomy, and capacity, and accordingly must never be permitted (19). Regardless of which side of the debate psychiatrists find themselves on, it seems as though the laws governing MAID are changing, and psychiatrists may increasingly find themselves asked to assess MAID eligibility for patients with mental illness who are not at end of life.
Summary
Providers who participate in DWD or MAID programs should be aware of the legal limitations to eligibility in their jurisdiction. In particular for patients with primary or metastatic brain tumors, providers should carefully consider and assess whether their patients have cognitive, psychiatric or mood issues that may require additional caution in certifying eligibility. Some of these issues may be correctible, especially if they are related to medications commonly used in this patient population like corticosteroids and antiepileptics, or temporarily induced by sleep disruptions, seizures, delirium or acute mood disorders related to the diagnosis and treatment (see accompanying article in this edition by Gibson et al.). In addition, in most jurisdictions providers certify patients eligibility for a future undetermined time of administration, so they would be unable to accurately assess if or when the patients eligibility might change after that time point. Prognostication is notoriously difficult at the individual patient level and may be even more so in patients with brain tumors (see accompanying article in this edition by Sharma et al.). Furthermore, little work has been done to assess how participation in these programs affects caregivers and providers, especially in terms of bereavement and issues related to spirituality (see accompanying articles in this edition by Fitchett et al. and Morris et al.). Clearly, more work is needed to help address these gaps in knowledge and guide providers, patients and caregivers as these programs have expanded to wider implementation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Palliative Medicine for the series “Palliative Care in Neuro-Oncology”. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-756
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-756). The series “Palliative Care in Neuro-Oncology” was commissioned by the editorial office without any funding or sponsorship. JJG served as the unpaid Guest Editor of the series. EIG reports personal fees from Celgene, USA, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oregon State Department of Health. Death with Dignity Act 2018 Data Summary. Available online: [Accessed on March 7th, 2020].https://www.oregon.gov/oha/PH/PROVIDERPARTNERRESOURCES/EVALUATIONRESEARCH/DEATHWITHDIGNITYACT/Documents/year21.pdf
- California State Department of Health. California End of Life Option Act 2018 Data Report. Available online: [Accessed on March 7th, 2020].https://www.deathwithdignity.org/wp-content/uploads/2019/08/CA-CDPH-End-of-Life-Option-Act-Report-2018.pdf
- Washington State Department of Health. 2018 Death with Dignity report. Available online: [Accessed on March 7th, 2020].https://www.doh.wa.gov/Portals/1/Documents/Pubs/422-109-DeathWithDignityAct2018.pdf
- Wang LH, Elliott MA, Henson LJ, et al. Death with dignity in Washington patients with amyotrophic lateral sclerosis. Neurology 2016;87:2117-22. [Crossref] [PubMed]
- Gofton TE, Graber J, Carver A. Identifying the palliative care needs of patients living with cerebral tumors and metastases: a retrospective analysis. J Neurooncol 2012;108:527-34. [Crossref] [PubMed]
- Ijzerman-Korevaar M, Snijders TJ, de Graeff A, et al. Prevalence of symptoms in glioma patients throughout the diseas trajectory: a systematic review. J Neurooncol 2018;140:485-96. [Crossref] [PubMed]
- Kim SY, Marson DC. Assessing decisional capacity in patients with brain tumors. Neurology 2014;83:482-3. [Crossref] [PubMed]
- Kerrigan S, Erridge S, Liaquat I, et al. Mental incapacity in patients undergoing neuro-oncologic treatment: a cross-sectional study. Neurology 2014;83:537-41. [Crossref] [PubMed]
- Sizoo EM, Pasman HR, Buttolo J, et al. Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer 2012;48:226-32. [Crossref] [PubMed]
- Marson DC, Martin RC, Triebel KL, et al. Capacity to consent to research participation in adults with malignant glioma. J Clin Oncol 2010;28:3844-50. [Crossref] [PubMed]
- Triebel KL, Gerstenecker A, Meneses K, et al. Capacity of patients with brain metastases to make treatment decisions. Psychooncology 2015;24:1448-55. [Crossref] [PubMed]
- Yager J, Ganzini L, Nguyen DH, et al. Working with decisionally capable patients who are determined to end their own lives. J Clin Psychiatry 2018;79:17r11767.
- Komrad MS, Pies RW, Hanson AL, et al. Assessing competency for physician-assisted suicide is unethical. J Clin Psychiatry 2018;79:18lr12566.
- Russell JA, Epstein LG, Bonnie RJ, et al. Lawful physician-hastened death. Neurology 2018;90:420-2. [Crossref] [PubMed]
- O’Rourke MA, O’Rourke MC, Hudson MF. Reasons to reject physician-assisted suicide/physician aid in dying. J Oncol Pract 2017;13:683-6. [Crossref] [PubMed]
- Spence RA, Blanke CD, Keating TJ, et al. Responding to patient requests for hastened death: Physician aid in dying and the clinical oncologist. J Oncol Pract 2017;13:693-9. [Crossref] [PubMed]
- Loggers ET, Starks H, Shannon-Dudley M, et al. Implementing a death with dignity program as a comprehensive cancer center. N Engl J Med 2013;368:1417-24. [Crossref] [PubMed]
- Statutes of Canada. Chapter 3, An Act to Amend the Criminal Code and to Make Related Amendments to Other Acts (Medical Assistance in Dying). Bill C-14. 2016. Available online: http://www.parl.ca/Content/Bills/421/Government/C-14/C-14_4/C-14_4.PDF.
- Dembo J, Schuklenk U, Reggler J. "For Their Own Good": A Response to Popular Arguments Against Permitting Medical Assistance in Dying (MAID) where Mental Illness Is the Sole Underlying Condition. Can J Psychiatry 2018;63:451-6. [Crossref] [PubMed]
- Council of Canadian Academies. The State of Knowledge on Medical Assistance in Dying Where a Mental Disorder Is the Sole Underlying Medical Condition. Ottawa, ON: The Expert Panel Working Group on MAID Where a Mental Disorder Is the Sole Underlying Medical Condition.; 2018.
- Appelbaum PS. Clinical practice. Assessment of patients' competence to consent to treatment. N Engl J Med 2007;357:1834-40. [Crossref] [PubMed]
- Oldham RL, Dobscha SK, Goy ER, et al. Attachment styles of Oregonians who request physician-assisted death. Palliat Support Care 2011;9:123-8. [Crossref] [PubMed]
- Kertesz A. Western Aphasia Battery-Revised. San Antonio, Tx: The Psychological Corporation. 2007.
- Government of Canada. Department of Justice. Medical Assistance in Dying: January 2020 Consultations. Available online: https://www.justice.gc.ca/eng/cons/ad-am/index.html


