
Radiotherapy to the brain: what are the consequences of this age-old treatment?
Introduction
Radiotherapy (RT) remains a cornerstone in the management of brain tumors. The use of RT has been described from as early as 1930s, when Lenz and Freid described ‘temporary regression of signs of increased intracranial pressure and localized brain involvement following moderate dosage of radiotherapy’ (1). Chao et al. in 1954 reported symptomatic relief in two-thirds of patients with brain metastases who received brain radiation (2). RT has since become an established treatment of brain tumors in the curative, palliative, and also prophylactic settings (as in the case of small cell lung cancer to reduce the incidence of brain metastases) (3). In the curative setting, RT is an important adjunct modality to surgery and chemotherapy in primary brain tumors such as gliomas as these tend to be infiltrative and are incompletely removed with surgery alone (4). In the setting of brain metastases, early trials have shown that whole-brain RT (WBRT) improved survival compared to corticosteroids alone (5,6). Up to 30% of cancer patients eventually develop brain metastases, and the goals of care in these patients have widened over the years to encompass preserving and improving quality of life. RT still has a major role to play, as the activity of systemic chemotherapy within brain parenchyma remains limited (7). However, in patients with poorer prognosis, WBRT may not offer significant benefit in terms of survival and quality of life. Therefore, the option of withholding WBRT may be considered (8).
The addition of local aggressive therapy (either surgery, or stereotactic radiosurgery) to WBRT lead to improved outcomes in patients with single or limited (1 to 3) brain metastases—including improved survival (in patients with a single lesion), fewer recurrence, and longer duration of functional independence (9-11). The use of SRS alone was then compared to upfront WBRT with SRS for patients with 1–4 intact brain metastases and no significant difference in survival was reported, however distant intracranial relapse was noted to be higher with SRS alone (12). The main drawback of adding WBRT to SRS for patients with limited brain metastases is the treatment-related neurocognitive deterioration which was first reported by Chang et al. in a single institution randomized controlled trial (RCT) in 2009 and more recently, in a larger scale multi-institution RCT (13,14). In 2014, the American Society for Radiation Oncology (ASTRO) recommended for WBRT to not be routinely added to SRS for limited brain metastases in their Choosing Wisely campaign and advised patients to undergo careful surveillance with consideration for salvage therapy in the event of relapse (15). Compared to WBRT, the use of SRS limits the volume of healthy brain parenchyma being exposed to radiation, however adjacent structures (such as cranial nerves, brainstem) are still at risk of developing complications. Stereotactic radiosurgery alone is now routinely recommended, by international guidelines, for patients with limited brain metastases, for it allows for better local control with fewer neurocognitive side effects (16,17). Table 1 shows the different modality and combination of treatment in the management of brain metastases.
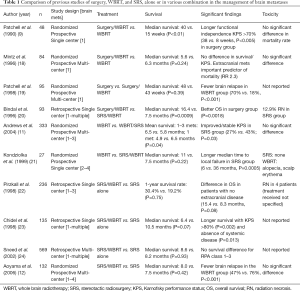
Full table
Nevertheless, careful patient selection to undergo RT is important. Late toxicities from RT can be debilitating, affect quality of life and at times irreversible. This may be more critical for those receiving RT for benign conditions (e.g., pituitary adenoma, meningioma) or prophylactically. Even in the palliative setting, patients are living longer due to improvements in systemic therapy. As such, prognostic tools form an important part of clinical decision making. Within the context of brain metastases, the Graded Prognostic Assessment (GPA) provides a histology-specific scoring system for prognosticating patients’ expected survival. This is based on factors including age, Karnofsky performance status (KPS), number of brain metastases and status of extracranial metastases (25). The Radiation Therapy Oncology Group (RTOG) evaluated three consecutive trials involving brain metastases in patients and used recursive partitioning analysis (RPA) to subdivide prognosis into 3 classes; class I (KPS >70, <65 years old, controlled primary, no extracranial metastases), class III (KPS <70), class II (all others) (26). An individualized prognostic nomogram for patients with brain metastases has been developed using de-identified data from 7 RTOG randomized clinical trials and is useful in counselling patients with regards to their prognosis (27).
In this review article, we will be looking closer into the effects of radiation on the brain, with a focus on late adverse events related to WBRT or SRS. We will briefly touch on the pathophysiology, clinical manifestation and mitigation strategies.
Radiation-related complications
Brain parenchyma is known to be a late-reacting tissue with a low alpha/beta ratio and a limited capacity to for repair (28). In addition, brain parenchyma exhibits a volume effect—where small volumes can tolerate higher radiation doses (29). The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) workgroup document is an important and widely referenced for organ-specific tissue tolerance to RT and will be included in relevant sections below (30). Based on the time of onset of clinical manifestation, radiation-related complications can be described as acute (occurring during or days to weeks after RT), early-delayed (few weeks to months after RT), or late (several months to years after RT) (31). Histologically, Szeifert et al. described 3 types of tissue response seen in post-SRS resected brain metastases; acute, subacute-, and chronic-type reactions. The acute-type, observed from 1–17 months post-SRS, is characterized by sharply demarcated coagulation necrosis. During the subacute type, observed from 5–59 months post-SRS, well circumscribed coagulation necrosis was observed, and in the chronic-type, observed from 9–33 months post-SRS, scar tissue and calcification was seen (32,33).
Acute
Cerebral edema is commonly encountered within days to weeks of RT to the brain and is due to radiation-induced vascular injury causing a transient increase in permeability (31). Clinically, it manifests as headache, nausea, or even worsening of pre-existing neurological deficits. Symptomatic edema was reported to occur in 5–43% of patients with meningioma after SRS (34). Corticosteroids typically provides good symptomatic relief, however there is no standardized guideline on corticosteroid prescription in the prevention or treatment of cerebral edema and the practice is largely physician dependent with low dose dexamethasone of 4–8 mg for 3–7 days prescribed with proton pump inhibitor being the most common practice (35,36). Radiation-induced seizures, particularly with SRS, can occur with 1–3 days post-SRS and may be more common for lesions located in the motor cortex (37). However, there remains a large variation in practice with regards to seizure prophylaxis for patients undergoing SRS (36). Fatigue is another well-known acute effect of WBRT (38). Alopecia, which may potentially cause distress in some patients, also frequently occurs in patients undergoing WBRT. A dose dependent effect has previously been reported with lower doses causing reversible alopecia with complete hair regrowth within 2–4 months whereas higher dose of RT causes irreversible alopecia (39,40). This has led to investigators evaluating scalp-sparing technique using intensity modulated radiation therapy (IMRT) (41).
Early delayed
Radiation-related demyelination has been implicated in the subacute phase of radiation-related complications which occur 1 to 6 months after RT. Although the tumor itself can induce demyelination in surrounding tissues due to compression and vascular disturbance, this is further contributed by radiation and chemotherapy (42). Interestingly, dose-dependent demyelination appears to occur early in areas receiving high RT doses and subsequent dose-independent demyelination occur 4–6 months after RT (43).
Neuropraxia
Neuropraxia may occur after SRS and is generally transient. For example, Chopra et al. reported trigeminal neuropathy 5–48 months following SRS for acoustic schwannoma in 4% of patients. About half of them only developed transient numbness and none developed facial palsy (44). This is consistent with findings from a retrospective study of 383 patients with SRS-treated vestibular schwannomas by Hansasuta et al. They reported hemifacial spasm in 2% of patients after SRS, half of which was transient, and none developed facial weakness post-SRS (45). However, a study of 162 patients who received SRS for acoustic neuromas reported normal facial and trigeminal function in 79% and 73% of patients, respectively, after 5 years (46). A more recent study of 49 patients receiving SRS for intracanalicular acoustic neurinoma reported Common Terminology Criteria for Adverse Events (CTCAE) grade 1 facial nerve disorder 3 months after SRS which resolved 3 months later, and one had CTCAE grade 2 facial muscle weakness which resolved 12 months later (47).
Somnolence syndrome
Somnolence syndrome can occur in up to 79% of patients following RT to the brain and is characterized by a combination of symptoms such as lethargy, clumsiness, reduced cognitive function, drowsiness, some of which overlap with symptoms of fatigue experienced by patients with cancer (48-50). In a prospective study involving 19 patients receiving high doses of RT for primary brain tumor, all experienced at least grade 1 tiredness based on the Littman scale and 84% developed > grade 2 somnolence symptoms (48). Subsequent larger study by the same group, of 70 patients undergoing radical RT for primary brain tumor, reported 90% of patients with grade 1 somnolence using the Littman score, which correlated with the visual analogue scale (VAS) score. A significant increase in score between week 3 and 12 was observed with a peak at the end of RT and improvement noticed from week 6 onwards (51). Of note, the Littman scale is a specific grading system for somnolence syndrome ranging from grade 0 (no change in behavior) to grade 4 (inactive, sleeping 18–20 hours a day with low grade fever, marked reduced appetite, and taking oral fluids only) (52). As somnolence syndrome can reduce patients’ functional ability and disrupt their daily routine, they should be fully informed of the likelihood that somnolence syndrome affects most patients undergoing RT for primary brain tumor with an estimated peak at 6 to 8 weeks after commencing RT and complete resolution 4 to 6 weeks later (51).
Late
Late complications usually occur more than 6 months after RT, tend to be irreversible and often progressive. The pathogenesis of late complications is often seen in the white matter and are linked to persistent demyelination, reduced neurogenesis with altered neural stem cell differentiation, inflammatory response through oxidative damage and disruption of microvasculature resulting in ischaemia and toxic neuro-excitation (53).
Radiation necrosis (RN)
RN typically occurs between 6–24 months after RT, however can present earlier in the re-treatment setting (54).
Risk factors
Risk factors for RN include re-irradiation (prior WBRT or SRS), SRS dose prescription, target volume, and location. Previous studies have reported a 10% risk of radiation necrosis with SRS (29,55). Large lesions (>4 cm diameter) are at a higher risk of developing RN when treated with SRS. Therefore, such cases may be better managed with upfront surgery followed by cavity irradiation, or fractionated stereotactic radiotherapy (FSRT) (56). A recent study comparing 1- and 3-fraction SRS with RN as the primary endpoint is summarized in Table 2. The risk of RN increases when the volume of normal brain parenchyma receiving 12 Gy or higher (in a single fraction) exceeds 10 cc, or when the volume receiving 30 Gy (in 5 fractions) or higher exceeds 10 cc (62,63). In particular, the risk of RN with repeat SRS has been reported to be 20% at 1 year and 4–8% with prior use of WBRT or WBRT used in conjunction with SRS (64). The preferred time interval for re-irradiation to the brain is still unclear, however measures such as minimizing PTV margin, optimizing patient setup and the use of image-guidance help to reduce the volume of normal brain parenchyma that is exposed to high doses of radiation (65).
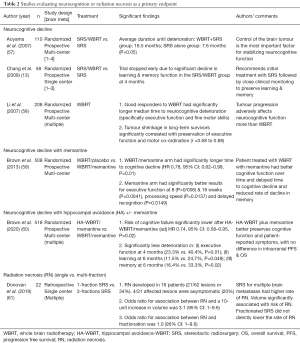
Full table
Concurrent systemic treatment with immunotherapy or targeted therapy may result in higher rate of post-SRS radiation necrosis (66,67). In a study by Kim et al., the use of concurrent targeted therapy (defined as administration within five biological half-lives) increased the 12-month cumulative incidence of radiological RN (8.8% vs. 5.3%, P<0.01) (68). This was particularly pronounced with VEGFR tyrosine kinase inhibitors (TKI) and EGFR TKIs. Concurrent chemotherapy increases the risk of RN in both primary brain and metastatic tumours (68,69). However, there is no standardized recommendation with regards to the ideal washout period between the use of chemotherapy and the delivery of SRS which is often decided on a case-by-case basis depending on the burden of systemic disease. Previous studies have also suggested that some locations within the brain are more prone to developing RN (such as the frontal cortex), whereas other locations (e.g., brainstem) are more resistant (70). Ohtakara and colleagues suggested that superficial lesions were at a lower risk of RN, as the dose spillage happens within non-brain parenchymal tissue (such as skull bone, skin) compared to deeper lesions (71).
Radiological features
Radiation necrosis may be difficult to distinguish from intra-cranial recurrences clinically and radiologically (55). Clinical signs largely depend on its size/location, or at times may remain asymptomatic. It appears as a contrast enhancing lesion (on T1 sequence) with surrounding edema and changes in signal intensity on MRI brain which is also a common feature of a recurrence (72). Surgical biopsy or resection of enlarging lesions post-SRS seen confirmed radiation necrosis in 22 out of 23 cases (73). Diffusion-weighted MR imaging has been used to distinguish between radiation necrosis and tumor progression (74). Amino-acid tracers (such as Carbon-11 methionine, and Fluoroethyltyrosine) in positron-emission tomography (PET) scanners are particularly useful, as normal brain parenchyma has a relatively lower amino acid uptake (75). For example, FET-PET imaging has been reported to have a sensitivity of 100% and specificity of 93% in the setting of recurrent gliomas (76). Additionally, MRI sequences such as Chemical Exchange Saturation Transfer (CEST) have shown promise in differentiating RN from tumour progression (77). Figure 1 shows the radiological features of RN seen on various imaging modalities and comparison with tumour progression (78).
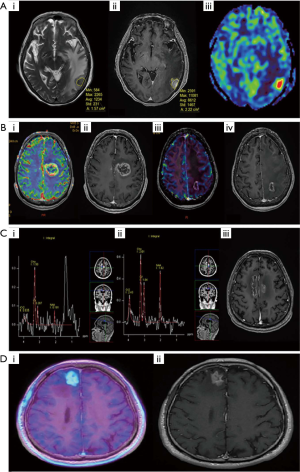
Pathophysiology
A retrospective study involving 516 brain metastases treated with gamma knife SRS (GK-SRS) reported increasing size of lesions in one third of brain metastases from 6 weeks to 15 months following GK-SRS. Ten patients underwent salvage resection and were found to have radiation necrosis appearing as inflammatory infiltrate with central necrosis on histopathological evaluation (73). This complex process is thought to be largely due to a combination of direct glial/oligodendrocyte injury, and immune-mediated perivascular infiltration of T-lymphocytes leading to cytokine release amongst others, and endothelial cell injury with blood brain barrier damage leading to increased permeability of the capillary network and basement membrane (31,79). These changes contribute to extracellular edema leading to focal neurological deficit (38,73,80,81).
Management
Making the diagnosis of radiation necrosis can be challenging but is a crucial part of management. Asymptomatic patients are usually managed with close observation and serial imaging. Those with symptoms may be treated with corticosteroids however potential side effects need to be carefully considered. Previous studies have shed light on the role of vascular endothelial growth factor (VEGF) therapy in promoting capillary permeability and evidence of VEGF overexpression in radiation necrosis (82,83). This has led to the development of the VEGF inhibitor bevacizumab in the treatment of radiation necrosis with one study reporting 64% reduction in the size of radiation necrosis at the first MRI follow-up (mean 26 days), reduced dose of steroids required, and improvement or stability in symptoms in 10 out of 11 of their patients (84). A pooled analysis of 71 patients showed that the use of bevacizumab provided patients with ~80% clinical improvement, with nearly all patients having radiographic response (85). Symptomatic patients refractory to medical treatment can be considered for surgery, however anesthesia and surgical risks have to be carefully weighed against its benefits (86). Magnetic resonance imaging (MRI)-guided laser-induced thermal therapy (LITT) is a well-tolerated minimally invasive procedure using laser light and heat to target tumor cells and peri-necrotic gliosis zone (87). Hyperbaric oxygen is an alternative option for patients not suitable for medical or surgical intervention and functions by enhancing angiogenesis in hypoxic or necrotic tissue (88).
Neurocognitive changes
Several studies have reported neurocognitive decline occurring weeks to months following WBRT. However, disease progression itself could also contribute to neurocognitive decline. A prospective study by Welzel et al. comparing cognitive function during and after RT in patients receiving prophylactic or therapeutic WBRT reported cognitive dysfunction 6 to 8 weeks after WBRT regardless of whether or not they had brain metastases (89). An RTOG trial evaluating 182 patients with unresectable brain metastases treated with WBRT reported pre-treatment MMSE as a statistically significant factor for survival and decreased risk of death with increased MMSE (90). They reported MMSE of >23 in 81% of patients at 6 months and 66% at 1 year. However, MMSE is not an optimal test for detecting neurocognitive deficits and more recent trials have used more sensitive tests such as the Hopkins Verbal Learning Test (HVLT). Table 2 shows selected studies of RT to the brain with neurocognition as its primary endpoint.
In a randomized controlled trial of patients with 1–4 brain metastases, Aoyama et al. found statistically significant difference in baseline mini mental state examination (MMSE) analyses on stratifying total tumor volume, extent of edema, age, and Karnofsky performance. Interestingly, they found the mean duration of time until cognitive deterioration was 16.5 months for the WBRT with SRS group compared to 7.6 months for the SRS alone group (P=0.05) (57). An RTOG trial evaluating 182 patients with unresectable brain metastases treated with WBRT reported pre-treatment MMSE as a statistically significant factor for survival and decreased risk of death with increased MMSE (90). They reported MMSE of >23 in 81% of patients at 6 months and 66% at 1 year. In a study comparing accelerated fractionation (AF) WBRT with 3 Gy daily treatment to 30 Gy vs. accelerated hyperfractionation (AH) WBRT with 1.6 Gy twice daily treatment to 54.4 Gy for unresectable brain metastases, Regine et al. reported no significant difference in MMSE between those receiving AF- or AH-WBRT, however observed a significantly lower MMSE score in those with uncontrolled brain metastases at 3 months post-WBRT (average MMSE decline of 0.05 in radiologically controlled brain metastases vs. 6.3 for those with uncontrolled brain metastases, P=0.02). These findings indicate that control of brain metastases has a significant role in neurocognitive function (91).
A large randomized clinical trial (the N0574 study) reported significantly less cognitive deterioration and higher quality of life at 3 months in 213 patients with 1 to 3 brain metastases receiving SRS alone compared to SRS with WBRT (14). A phase II trial solely focusing on health-related quality of life (HRQOL) reported worse HRQOL in patients who received adjuvant WBRT and recommended observation after initial surgery or SRS for limited brain metastases (92).
Measures to reduce risk of neurocognitive decline
Radiation injury to the hippocampal neural stem cells affects neurocognitive function in many aspects, including verbal and non-verbal memory, executive function, attention span and information processing speed (93,94). Studies have consistently demonstrated benefits of hippocampal avoidance in preserving cognitive function. A prospective study of 53 patients with primary brain tumor treated with conventional fractionated RT reported hippocampal V53.4 Gy to V60.9 Gy (i.e., percentage volume receiving 53.4 to 60.9 Gy) as a statistically significant predictor of memory impairment following RT with V55 Gy as being the most significant predictor of neurocognitive decline (95). In a phase II trial evaluating hippocampal-sparing WBRT (using IMRT) 30 Gy in 10 fractions for brain metastases in 113 patients (RTOG 0933), significant preservation of memory and quality of life was observed compared to historical control (96). A retrospective study of hippocampal-sparing RT to primary brain tumor using volumetric modulated arc therapy (VMAT) reported that the contralateral hippocampus could be reasonably spared to preserve verbal memory function. Interestingly, decline in memory function was associated with the left hippocampal mean dose and was not associated with the right hippocampal mean dose (97). Most recently, a phase III trial comparing hippocampal-avoidance WBRT (HA-WBRT) plus memantine or conventional WBRT plus memantine reported significantly lower risk of cognitive failure with HA-WBRT plus memantine (adjusted HR 0.74; 95% CI: 0.58–0.95, P=0.02). The HA-WBRT plus memantine group was observed to have better preserved executive function (at 4 months), learning, and memory (at 6 months). They were also found to have less fatigue, less difficulty with speech, and less interference of neurologic symptoms in daily activities (60). This can now be considered the standard of care in patients with brain metastases, not eligible for SRS and have a prognosis of at least 4–6 months.
Memantine is an N-methyl-D-aspartate (NMDA) receptor antagonist previously shown to reduce clinical deterioration in moderate to severe Alzheimer’s dementia through its anti-glutamatergic effect in the brain as overstimulation of the NMDA receptor by glutamate contributes to the development of neurodegenerative disorders (98). In a randomized, double-blind, placebo-controlled trial of memantine in patients with brain metastases receiving WBRT to a total dose of 37.5 Gy in 15 fractions, patients were assigned to either the placebo or memantine which was given as an escalating dose regimen up to 20 mg/day started within 3 days of initiating RT for 24 weeks. The probability of cognitive function failure at 24 weeks was 53.8% in the memantine arm vs. 64.9% in the placebo arm; HR 0.78 95% CI: 0.62–0.99, P=0.01), indicating that memantine significantly delayed time to cognitive decline. Patients receiving memantine were also found to have significantly better executive function at 8 and 16 weeks, as well as processing speed and delayed recognition at 24 weeks (59). In the same study, the rate of cognitive decline was reported to have slowed by 4 months post-RT in both arms and this was more pronounced in the memantine arm. However, we must not forget that cognitive function is multifactorial, and can be affected by both disease progression and cancer treatments such as radiotherapy and chemotherapy (58). As such, opting for the treatment with the least neuro-cognitive toxicity to provide intra-cranial disease control would be in our best interest to maintain a reasonable quality of life.
Brainstem injury
Radiation injury to the brainstem can result in cranial nerve III to XII neuropathies depending on the exact location affected and can lead to profound and permanent neurological deficit with potential life-threatening effects on the cardiovascular and respiratory systems. Significant RT-related brainstem injury can occur months to years after RT and can be challenging to distinguish from disease progression (99). The CTCAE is used to grade the severity of each cranial nerve injury—from mild or asymptomatic (grade 1), moderate and limiting instrumental ADL (grade 2), severe symptoms limiting ADL (grade 3), life threatening consequences (grade 4), death (grade 5) (100). The QUANTEC analysis recommends a maximum dose of 54 Gy to the whole brainstem using conventional fractionation of photon RT and higher dose limit of 59 Gy for smaller volumes of the brainstem (1–10 mL) (99). Due to its potential detrimental effects, brainstem dose constraints tend to be prioritized over tumor coverage, and hence overall incidence of brainstem injury is low. Previous studies have reported relatively low complication rates with 15–20 Gy of SRS in patients with poor prognosis (101,102). For single fraction SRS, QUANTEC recommends a maximum dose of 12.5 Gy to the brainstem whereas the (AAPM) Task Group 101 recommends a maximum point dose of 15 Gy (99,103). Improving accuracy during contouring and planning using high-resolution magnetic resonance imaging (MRI) images will help to reduce unnecessary toxicity to surrounding tissue.
Cranial nerves injury
Optic neuropathy
Radiation-related optic neuropathy (RON) results in painless irreversible visual loss with the majority occurring within 3 years post-RT (peak incidence of 1–1.5 years) (104). Clinical signs are determined by the exact site of injury such that injury to the optic nerve leads to ipsilateral monocular vision loss, whereas injury to the whole optic chiasm leads to bilateral vision loss. Disruption to the decussating fibers at the central chiasm typically features as bitemporal hemianopia, and damage to the optic tract leads to homonymous hemianopia of the contralateral eye (105,106). On MRI imaging, contrast-enhancement on T1 and high signal T2 change, is usually seen in the pre-chiasmatic portion of the optic nerve (107). A QUANTEC analysis on radiation dose-volume to optic nerves and chiasms concluded that the risk of toxicity significantly increased at doses >60 Gy at ~1.8 Gy/fractions for fractionated RT and >12 Gy for SRS (106,107). Milano et al. reported single fraction 10 Gy to be associated with 1% risk of RON (108). However, it is interesting to note that some patients remain asymptomatic, despite imaging and ophthalmologic findings.
Previous studies have reported the risk of RON is ~1% for patients receiving up to 12 Gy (109), whereas doses exceeding 12 Gy lead to a 10% risk (110). Whether underlying vasculopathy such as diabetes mellitus or hypertension contribute to RON remains controversial (107,111). Measures to reduce the risk of RON include using high-resolution magnetic resonance imaging (MRI) images to aid contouring, appropriate dose selection, and optimizing the plan.
Management of RON is challenging and only achieves limited benefit with various treatments including steroids, vitamin E, pentoxifylline. The anti-vascular endothelial growth factor (anti-VEGF) monoclonal antibody bevacizumab was reported to result in improved or stabilized visual acuity in the majority of patients in a case series of 14 patients with RON receiving intravenous bevacizumab (112). Hyperbaric oxygen therapy may possibly help if initiated within 72 hours of the injury however benefit may be limited to temporary partial relief and the treatment is delivered over multiple sessions which may not be convenient for patients (113,114).
Damage to III, IV, V, VI nerves
The above cranial nerves (CN) are able to tolerate higher doses of single fraction SRS better than the optic nerve, such that in a series of 1255 patients with pituitary adenoma who were treated with SRS (14–34 Gy), only 0.4% had a permanent deficit of CN III, IV, VI and only 0.2% had a deficit of CN V (115). Previous HSRT studies suggest the tolerance of these nerves in 3 fractions to be 21 Gy (116,117).
Damage to the VIII nerve and cochlea
Radiation injury to the cochlea and vestibulocochlear (VIII) nerve results in sensorineural hearing loss (SNHL) which occurs months to years after RT and typically features as impaired hearing at the high frequency range on pure-tone audiometry (118,119). A prospective study of 294 patients, of which 526 ears were eligible to be included, reported deterioration in bone conduction threshold at 4 kHz in 31% of patients and pure tone average in 14% of patients within 3 months after RT for nasopharyngeal carcinoma (NPC). The same study reported age >50 years and ears with threshold below 60 dB at 4 kHz before RT to be factors significantly associated with a 4 kHz hearing loss (119). At 2-year follow-up, significant recovery was reported in 37% of ears (more than 10 dB recovery at both 4 kHz and pure-tone audiometry), however at 4.5-year follow-up in 74 ears, significant deterioration was more evident (119). Concurrent chemotherapy with platinum-based agents collectively worsens hearing loss with several studies reporting a dose-related effect with cisplatin (120,121). QUANTEC recommends the mean dose of the cochlea to be limited to <45 Gy to keep SNHL below 30% (122). Treatment such as corticosteroids (to reduce inflammation and edema in the inner ear), hyperbaric oxygen (to promote regeneration capabilities), and classical air conduction hearing aids have been tried with mixed results. Cochlear implants have had promising results however are not always helpful in RT-induced hearing loss as injury to radiation injury to the vestibulocochlear nerve (123,124).
Effects on the hypothalamus/pituitary axis
Early studies postulated radiation-induced pituitary dysfunction to be the result of hypothalamic damage resulting in loss of the hypothalamic releasing hormones rather than actual damage to the pituitary itself which was thought to be relatively radioresistant (125-127). Subsequent studies have demonstrated higher radiation dose increases the risk of both hypothalamic and pituitary insufficiencies and this occurred in a time-dependent manner (i.e., more prevalent with longer follow-up post-RT) (128). A meta-analysis evaluating pituitary dysfunction ~1–20 years after cranial RT in adults reported a prevalence of 0.66 (95% CI: 0.55–0.76) for any degree of hypopituitarism (0.54; 95% CI: 0.42–0.66) post-RT for brain tumors and 0.74 (95% CI: 0.57–0.86) post-RT for nasopharyngeal tumors). Growth hormone (GH) deficiency was the most prevalent (0.45; 95% CI: 0.33–0.57), followed by luteinizing hormone & follicle stimulating hormone (0.3; 95% CI: 0.23–0.37), thyroid stimulating hormone (0.25; 95% CI, 0.16–0.37), adrenocorticotropin stimulating hormone (0.22; 95% CI: 0.15–0.3) (129). Isolated GH deficiency has been observed to occur with lower doses of RT (<30 Gy) whereas higher doses will affect other pituitary hormones (128,130). With studies reporting pituitary hormone deficiency detected as late as 26 years post-RT, pituitary function should be routinely assessed during follow-up of patients after RT to the brain and head & neck region where the hypothalamic, pituitary and thyroid glands are within the RT field (131).
Stroke
Cerebrovascular events (CVE) is a late complication that can occur many years after RT. Atherosclerosis, which has long been attributed as a major cause of stroke, is a known complication of RT (132). In a study of patients receiving primary treatment for craniopharyngioma, the rate of clinically apparent CVE at 10 years was 11% (15% for those receiving higher dose of RT (EQD2 >50 Gy) and 8% for those receiving lower dose of RT (EQD2 <50 Gy), P=0.3). Although this difference was not statistically significant, other studies with longer follow-up have demonstrated significant correlation between increased radiation dose and risk of CVE. One study of pediatric cancer survivors with a mean follow-up of 23.3 years reported increased risk of CVE in a dose-dependent manner with hazard ratio (HR) 5.9 (95% CI: 3.5–9.9) for 30–49 Gy and HR 11.0 (7.4–17.0) for >50 Gy (133). Of note, EQD2 is the equivalent dose in 2 Gy fractions, derived by using a formula to convert the total radiation dose to EQD2 (134). The same study reported a cumulative incidence of stroke of 1.1% (95% CI: 0.4–1.8) for patients who received >50 Gy of cranial RT at 10 years post-diagnosis and 12% (95% CI: 8.9–15.0) at 30 years post-diagnosis (133). As such, managing modifiable risk factors, which contribute to CVE, such as tobacco use, diabetes, hypertension, hyperlipidemia become important in the long-term follow-up of these patients.
Secondary malignancy
Radiation-induced secondary malignancy is a potential long-term complication occurring many years after RT and diagnostic criteria include tumors that occur within the irradiated field, adequate latency period, histologically different from the primary tumor, no other associated pathology present e.g., neurofibromatosis (135). Meningioma and glioma are the most widely reported secondary malignancy occurring after cranial RT. Studies have reported cumulative incidence of secondary brain malignancy as 2.7% at 15 years and 2.4% at 20 years (136,137). In a meta-analysis of 296 cases of secondary glioma post-RT, mean latency period between RT and diagnosis of any grade of secondary glioma was 9 years (95% CI: 8–9.5). Interestingly, they observed that those who received systemic chemotherapy had a mean latency period of 8 years (95% CI: 7–9) and those without chemotherapy had a mean latency period of 10 years (95% CI: 9–12, P<0.0001) (138).
Conclusions
RT continues to play an essential role in the management of primary and metastatic brain tumors; however, the risks and benefits have to be thoroughly considered and fully discussed with patients as it can significantly affect their quality of life and daily function. As intracranial disease progression also contributes to neurocognitive and functional decline, each case should be carefully evaluated and the most appropriate modality of treatment (considering clinical indication, expected prognosis, associated risk factors, toxicity, and cost). Advances in brain imaging have aided radiological diagnosis and improved the accuracy of delineating tumors and critical structures help to reduce potential complications. Other measures to reduce complications, such as with memantine, hippocampal sparing WBRT or use of SRS should be utilized in suitable patients where possible. Increasing awareness of potential RT-related complications will allow patients to be managed appropriately and although most late effects tend to be irreversible, treatment to help alleviate specific symptoms or measures to aid patients’ daily function can make a difference to their lives and should be initiated early.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jerome Graber, Hany Soliman) for the series “Palliative Care in Neuro-Oncology” published in Annals of Palliative Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-856). The series “Palliative Care in Neuro-Oncology” was commissioned by the editorial office without any funding or sponsorship. SSL serves as an unpaid associate editor-in-chief of Annals of Palliative Medicine from Dec 2019 to Nov 2021. STC reports personal fees from Varian Medical Systems, outside the submitted work. SSL reports other from Elekta AB, outside the submitted work. AS is an advisor/consultant with Abbvie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board), a board member of International Stereotactic Radiosurgery Society (ISRS) and belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia. AS also reports past educational seminars with Elekta AB, Accuray Inc., Varian (CNS Teaching Faculty), BrainLAB, Medtronic Kyphon, and research grant with Elekta AB, and travel accommodations/expenses by Elekta, Varian, BrainLAB, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lenz M, Freid JR. Metastases to the skeleton, brain and spinal cord from cancer of the breast and the effect of radiotherapy. Ann Surg 1931;93:278-93. [Crossref] [PubMed]
- Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer 1954;7:682-9. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Walker MD, Alexander E, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 1978;49:333-43. [Crossref] [PubMed]
- Weissman DE. Glucocorticoid treatment for brain metastases and epidural spinal cord compression: a review. J Clin Oncol 1988;6:543-51. [Crossref] [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [Crossref] [PubMed]
- Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 2006;24:1295-304. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000;47:291-8. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- (ASTRO), T.A.S.f.R.O. Choosing Wisely. 2014 [cited 2020 8 February]; Available online: https://www.choosingwisely.org/astro-releases-second-list/.
- Network Comprehensive Cancer Network (NCCN) Central Nervous System Cancers. Clinical Practice Guidelines in Oncology 2020 [cited 2020 8 March]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- Chao ST, De Salles A, Hayashi M, et al. Stereotactic Radiosurgery in the Management of Limited (1-4) Brain Metasteses: Systematic Review and International Stereotactic Radiosurgery Society Practice Guideline. Neurosurgery 2018;83:345-53. [Crossref] [PubMed]
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78:1470-6. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [Crossref] [PubMed]
- Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg 1996;84:748-54. [Crossref] [PubMed]
- Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427-34. [Crossref] [PubMed]
- Pirzkall A, Debus J, Lohr F, et al. Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol 1998;16:3563-9. [Crossref] [PubMed]
- Chidel MA, Suh JH, Reddy CA, et al. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys 2000;47:993-9. [Crossref] [PubMed]
- Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys 2002;53:519-26. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Gaspar L, Scott S, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Yu C, Sloan AE, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol 2012;14:910-8. [Crossref] [PubMed]
- van Leeuwen CM, Oei AL, Crezee J, et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol 2018;13:96. [Crossref] [PubMed]
- Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys 2010;76:S20-7. [Crossref] [PubMed]
- Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 2010;76:S3-9. [Crossref] [PubMed]
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys 1980;6:1215-28. [Crossref] [PubMed]
- Szeifert GT, Atteberry DS, Kondziolka D, et al. Cerebral metastases pathology after radiosurgery: a multicenter study. Cancer 2006;106:2672-81. [Crossref] [PubMed]
- Szeifert GT, Kondziolka D, Levivier M, et al. Histopathology of brain metastases after radiosurgery. Prog Neurol Surg 2012;25:30-8. [Crossref] [PubMed]
- Milano MT, Sharma M, Soltys SG, et al. Radiation-Induced Edema After Single-Fraction or Multifraction Stereotactic Radiosurgery for Meningioma: A Critical Review. Int J Radiat Oncol Biol Phys 2018;101:344-57. [Crossref] [PubMed]
- Evans ML, Graham MM, Mahler PA, et al. Use of steroids to suppress vascular response to radiation. Int J Radiat Oncol Biol Phys 1987;13:563-7. [Crossref] [PubMed]
- Arvold ND, Pinnell NE, Mahadevan A, et al. Steroid and anticonvulsant prophylaxis for stereotactic radiosurgery: Large variation in physician recommendations. Pract Radiat Oncol 2016;6:e89-96. [Crossref] [PubMed]
- Gelblum DY, Lee H, Bilsky M, et al. Radiographic findings and morbidity in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 1998;42:391-5. [Crossref] [PubMed]
- Cross NE, Glantz MJ. Neurologic complications of radiation therapy. Neurol Clin 2003;21:249-77. [Crossref] [PubMed]
- Ali SY, Singh G. Radiation-induced Alopecia. Int J Trichology 2010;2:118-9. [Crossref] [PubMed]
- Severs GA, Griffin T, Werner-Wasik M. Cicatricial alopecia secondary to radiation therapy: case report and review of the literature. Cutis 2008;81:147-53. [PubMed]
- Witek M, Vahknenko Y, Siglin J, et al. Dose Reduction to the Scalp with Hippocampal Sparing Is Achievable with Intensity Modulated Radiotherapy. Int J Med Phys Clin Eng Radiat Oncol 2014;3:176-82. [Crossref] [PubMed]
- Helson L. Radiation-induced Demyelination and Remyelination in the Central Nervous System: A Literature Review. Anticancer Res 2018;38:4999-5002. [Crossref] [PubMed]
- Nagesh V, Tsien CI, Chenevert TL, et al. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys 2008;70:1002-10. [Crossref] [PubMed]
- Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2007;68:845-51. [Crossref] [PubMed]
- Hansasuta A, Choi CYH, Gibbs IC, et al. Multisession stereotactic radiosurgery for vestibular schwannomas: single-institution experience with 383 cases. Neurosurgery 2011;69:1200-9. [Crossref] [PubMed]
- Kondziolka D, Lunsford LD, McLaughlin MR, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 1998;339:1426-33. [Crossref] [PubMed]
- Rueß D, Pöhlmann L, Grau S, et al. Long-term follow-up after stereotactic radiosurgery of intracanalicular acoustic neurinoma. Radiat Oncol 2017;12:68. [Crossref] [PubMed]
- Faithfull S, Brada M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol (R Coll Radiol) 1998;10:250-4. [Crossref] [PubMed]
- Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist 2000;5:353-60. [Crossref] [PubMed]
- Dy SM, Lorenz KA, Naeim A, et al. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J Clin Oncol 2008;26:3886-95. [Crossref] [PubMed]
- Powell C, Guerrero D, Sardell S, et al. Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: a prospective study. Radiother Oncol 2011;100:131-6. [Crossref] [PubMed]
- Harjani RR, Gururajachar JM, Krishnaswamy U. Comprehensive assessment of Somnolence Syndrome in patients undergoing radiation to the brain. Rep Pract Oncol Radiother 2016;21:560-6. [Crossref] [PubMed]
- Wilke C, Grosshans D, Duman J, et al. Radiation-induced cognitive toxicity: pathophysiology and interventions to reduce toxicity in adults. Neuro Oncol 2018;20:597-607. [Crossref] [PubMed]
- Walker AJ, Ruzevick J, Malayeri AA, et al. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 2014;10:1277-97. [Crossref] [PubMed]
- Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist 2003;9:180-8. [Crossref] [PubMed]
- Lehrer EJ, Peterson JL, Zaorsky NG, et al. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J Radiat Oncol Biol Phys 2019;103:618-30. [Crossref] [PubMed]
- Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 2007;68:1388-95. [Crossref] [PubMed]
- Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol 2007;25:1260-6. [Crossref] [PubMed]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]
- Brown PD, Gondi V, Pugh S, et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol 2020;38:1019-29. [Crossref] [PubMed]
- Donovan EK, Parpia S, Greenspoon JN. Incidence of radionecrosis in single-fraction radiosurgery compared with fractionated radiotherapy in the treatment of brain metastasis. Curr Oncol 2019;26:e328-e333. [Crossref] [PubMed]
- Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996-1001. [Crossref] [PubMed]
- Faruqi S, Ruschin M, Soliman H, et al. Adverse Radiation Effect After Hypofractionated Stereotactic Radiosurgery in 5 Daily Fractions for Surgical Cavities and Intact Brain Metastases. Int J Radiat Oncol Biol Phys 2020;106:772-9. [Crossref] [PubMed]
- Sneed PK, Mendez J, Vemer-van den Hoek JGM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg 2015;123:373-86. [Crossref] [PubMed]
- Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys 2015;91:100-8. [Crossref] [PubMed]
- Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016;125:17-23. [Crossref] [PubMed]
- Patel KR, Chowdhary M, Switchenko JM, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res 2016;26:387-94. [Crossref] [PubMed]
- Kim JM, Miller JA, Kotecha R, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol 2017;133:357-68. [Crossref] [PubMed]
- Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 2006;65:499-508. [Crossref] [PubMed]
- Flickinger JC, Kondziolka D, Lunsford LD, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys 2000;46:1143-8. [Crossref] [PubMed]
- Ohtakara K, Hayashi S, Nakayama N, et al. Significance of target location relative to the depth from the brain surface and high-dose irradiated volume in the development of brain radionecrosis after micromultileaf collimator-based stereotactic radiosurgery for brain metastases. J Neurooncol 2012;108:201-9. [Crossref] [PubMed]
- Barajas RF, Chang JS, Sneed PK, et al. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2009;30:367-72. [Crossref] [PubMed]
- Patel TR, McHugh BJ, Bi WL, et al. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol 2011;32:1885-92. [Crossref] [PubMed]
- White NS, McDonald C, Farid N, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res 2014;74:4638-52. [Crossref] [PubMed]
- Miyake K, Shinomiya A, Okada M, et al. Usefulness of FDG, MET and FLT-PET studies for the management of human gliomas. J Biomed Biotechnol 2012;2012:205818. [Crossref] [PubMed]
- Rachinger W, Goetz C, Pöpperl G, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 2005;57:505-11. [Crossref] [PubMed]
- Mehrabian H, Desmond KL, Soliman H, et al. Differentiation between Radiation Necrosis and Tumor Progression Using Chemical Exchange Saturation Transfer. Clin Cancer Res 2017;23:3667-75. [Crossref] [PubMed]
- Vellayappan B, Tan CL, Yong C, et al. Diagnosis and Management of Radiation Necrosis in Patients With Brain Metastases. Front Oncol 2018;8:395. [Crossref] [PubMed]
- Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci 2013;20:485-502. [Crossref] [PubMed]
- Jagannathan J, Bourne TD, Schlesinger D, et al. Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery 2010;66:208-17. [Crossref] [PubMed]
- Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 2008;25:51-8. [Crossref] [PubMed]
- Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 1989;84:1470-8. [Crossref] [PubMed]
- Nonoguchi N, Miyatake SI, Fukumoto M, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol 2011;105:423-31. [Crossref] [PubMed]
- Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol 2013;15:1257-63. [Crossref] [PubMed]
- Tye K, Engelhard HH, Slavin KV, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neurooncol 2014;117:321-7. [Crossref] [PubMed]
- McPherson CM, Warnick RE. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol 2004;68:41-7. [Crossref] [PubMed]
- Rao MS, Hargreaves EL, Khan AJ, et al. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery 2014;74:658-67. [Crossref] [PubMed]
- Patel U, Patel A, Cobb C, et al. The management of brain necrosis as a result of SRS treatment for intra-cranial tumors. Transl Cancer Res 2014;3:373-82.
- Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 2008;72:1311-8. [Crossref] [PubMed]
- Murray KJ, Scott C, Zachariah B, et al. Importance of the mini-mental status examination in the treatment of patients with brain metastases: a report from the Radiation Therapy Oncology Group protocol 91-04. Int J Radiat Oncol Biol Phys 2000;48:59-64. [Crossref] [PubMed]
- Regine WF, Scott C, Murray K, et al. Neurocognitive outcome in brain metastases patients treated with accelerated-fractionation vs. accelerated-hyperfractionated radiotherapy: an analysis from Radiation Therapy Oncology Group Study 91-04. Int J Radiat Oncol Biol Phys 2001;51:711-7. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol 2010;97:370-6. [Crossref] [PubMed]
- McDuff SG, Taich ZT, Lawson JD, et al. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. J Neurol Neurosurg Psychiatry 2013;84:1384-91. [Crossref] [PubMed]
- Okoukoni C, McTyre ER, Peacock DNA, et al. Hippocampal dose volume histogram predicts Hopkins Verbal Learning Test scores after brain irradiation. Adv Radiat Oncol 2017;2:624-9. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Kim KS, Wee CW, Seok JY, et al. Hippocampus-sparing radiotherapy using volumetric modulated arc therapy (VMAT) to the primary brain tumor: the result of dosimetric study and neurocognitive function assessment. Radiat Oncol 2018;13:29. [Crossref] [PubMed]
- Reisberg B, Doody R, Stöffler A, et al. Memantine in Moderate-to-Severe Alzheimer's Disease. N Engl J Med 2003;348:1333-41. [Crossref] [PubMed]
- Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys 2010;76:S36-41. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. 2017 [cited 2020 18 February]; 5.0:[Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- Trifiletti DM, Lee CC, Kano H, et al. Stereotactic Radiosurgery for Brainstem Metastases: An International Cooperative Study to Define Response and Toxicity. Int J Radiat Oncol Biol Phys 2016;96:280-8. [Crossref] [PubMed]
- Leeman JE, Clump DA, Wegner RE, et al. Prescription dose and fractionation predict improved survival after stereotactic radiotherapy for brainstem metastases. Radiat Oncol 2012;7:107. [Crossref] [PubMed]
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [Crossref] [PubMed]
- Kline LB, Kim JY, Ceballos R. Radiation optic neuropathy. Ophthalmology 1985;92:1118-26. [Crossref] [PubMed]
- Celesia GG, DeMarco PJ. Anatomy and physiology of the visual system. J Clin Neurophysiol 1994;11:482-92. [Crossref] [PubMed]
- Mayo C, Martel MK, Marks LB, et al. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 2010;76:S28-35. [Crossref] [PubMed]
- Archer EL, Liao EA, Trobe JD. Radiation-Induced Optic Neuropathy: Clinical and Imaging Profile of Twelve Patients. J Neuroophthalmol 2019;39:170-80. [Crossref] [PubMed]
- Milano MT, Grimm J, Soltys SG, et al. Single- and Multi-Fraction Stereotactic Radiosurgery Dose Tolerances of the Optic Pathways. Int J Radiat Oncol Biol Phys 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Stafford SL, Pollock BE, Leavitt JA, et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2003;55:1177-81. [Crossref] [PubMed]
- Leavitt JA, Stafford SL, Link MJ, et al. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2013;87:524-7. [Crossref] [PubMed]
- Ferguson I, Huecker J, Huang J, et al. Risk factors for radiation-induced optic neuropathy: a case-control study. Clin Exp Ophthalmol 2017;45:592-7. [Crossref] [PubMed]
- Finger PT, Chin KJ. Antivascular endothelial growth factor bevacizumab for radiation optic neuropathy: secondary to plaque radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:789-98. [Crossref] [PubMed]
- Levy RL, Miller NR. Hyperbaric oxygen therapy for radiation-induced optic neuropathy. Ann Acad Med Singapore 2006;35:151-7. [PubMed]
- Guy J, Schatz NJ. Hyperbaric oxygen in the treatment of radiation-induced optic neuropathy. Ophthalmology 1986;93:1083-8. [Crossref] [PubMed]
- Witt TC. Stereotactic radiosurgery for pituitary tumors. Neurosurg Focus 2003;14:e10. [Crossref] [PubMed]
- Wang X, Liu X, Mei G, et al. Phase II study to assess the efficacy of hypofractionated stereotactic radiotherapy in patients with large cavernous sinus hemangiomas. Int J Radiat Oncol Biol Phys 2012;83:e223-30. [Crossref] [PubMed]
- Morimoto M, Yoshioka Y, Kotsuma T, et al. Hypofractionated stereotactic radiation therapy in three to five fractions for vestibular schwannoma. Jpn J Clin Oncol 2013;43:805-12. [Crossref] [PubMed]
- Jereczek-Fossa BA, Zarowski A, Milani F, et al. Radiotherapy-induced ear toxicity. Cancer Treat Rev 2003;29:417-30. [Crossref] [PubMed]
- Ho WK, Wei WI, Kwong DL, et al. Long-term sensorineural hearing deficit following radiotherapy in patients suffering from nasopharyngeal carcinoma: A prospective study. Head Neck 1999;21:547-53. [Crossref] [PubMed]
- Theunissen EAR, Zuur CL, Józwiak K, et al. Prediction of Hearing Loss Due to Cisplatin Chemoradiotherapy. JAMA Otolaryngol Head Neck Surg 2015;141:810-5. [Crossref] [PubMed]
- Schuette A, Lander DP, Kallogjeri D, et al. Predicting Hearing Loss After Radiotherapy and Cisplatin Chemotherapy in Patients With Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg 2020;146:106-12. [Crossref] [PubMed]
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9. [Crossref] [PubMed]
- Chua DY, Tan HK. Successful rehabilitation with cochlear implant in post-irradiation induced hearing loss in nasopharyngeal carcinoma patient. Ann Acad Med Singapore 2007;36:74-7. [PubMed]
- Formanek M, Czerny C, Gstoettner W, et al. Cochlear implantation as a successful rehabilitation for radiation-induced deafness. Eur Arch Otorhinolaryngol 1998;255:175-8. [Crossref] [PubMed]
- Arnold A. Effects of x-irradiation on the hypothalamus: a possible explanation for the therapeutic benefits following x-irradiation of the hypophysial region for pituitary dysfunction. J Clin Endocrinol Metab 1954;14:859-68. [Crossref] [PubMed]
- Lawrence AM, Pinsky SM, Goldfine ID. Conventional radiation therapy in acromegaly. A review and reassessment. Arch Intern Med 1971;128:369-77. [Crossref] [PubMed]
- Larkins RG, Martin FI. Hypopituitarism after extracranial irradiation: evidence for hypothalamic origin. BMJ 1973;1:152-153. [Crossref] [PubMed]
- Littley MD, Shalet SM, Beardwell CG, et al. Radiation-induced hypopituitarism is dose-dependent. Clin Endocrinol (Oxf) 1989;31:363-73. [Crossref] [PubMed]
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:2330-40. [Crossref] [PubMed]
- Costin G. Effects of low-dose cranial radiation on growth hormone secretory dynamics and hypothalamic-pituitary function. Am J Dis Child 1988;142:847-52. [PubMed]
- Samaan NA, Vieto R, Schultz PN, et al. Hypothalamic, pituitary and thyroid dysfunction after radiotherapy to the head and neck. Int J Radiat Oncol Biol Phys 1982;8:1857-67. [Crossref] [PubMed]
- Dorresteijn LD, Kappelle AC, Scholz NM, et al. Increased carotid wall thickening after radiotherapy on the neck. Eur J Cancer 2005;41:1026-30. [Crossref] [PubMed]
- Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys 2013;86:649-55. [Crossref] [PubMed]
- Lo AC, Howard AF, Nichol A, et al. Long-term outcomes and complications in patients with craniopharyngioma: the British Columbia Cancer Agency experience. Int J Radiat Oncol Biol Phys 2014;88:1011-8. [Crossref] [PubMed]
- Cahan WG, Woodard HQ, Higinbotham NL, et al. Sarcoma arising in irradiated bone: report of eleven cases. 1948. Cancer 1998;82:8-34. [Crossref] [PubMed]
- Tsang RW, Brierley JD, Panzarella T, et al. Radiation therapy for pituitary adenoma: treatment outcome and prognostic factors. Int J Radiat Oncol Biol Phys 1994;30:557-65. [Crossref] [PubMed]
- Minniti G, Traish D, Ashley S, et al. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab 2005;90:800-4. [Crossref] [PubMed]
- Yamanaka R, Hayano A, Kanayama T. Radiation-induced gliomas: a comprehensive review and meta-analysis. Neurosurg Rev 2018;41:719-31. [Crossref] [PubMed]


