Complete response to pembrolizumab in a patient with extensive-stage small-cell lung cancer: a case report
Introduction
Lung cancer is the most commonly diagnosed cancer in men and women. It is also the leading cause of cancer death worldwide, accounting for 18.4% of all cancer-related deaths. Small cell lung cancer (SCLC) comprises approximately 15% of lung cancer cases and is characterized by rapid growth and early extensive metastasis (1). Extensive-stage disease (ED-SCLC), defined as the presence of metastasis or extension beyond a single tolerable radiation port, accounts for 60–70% of all SCLC patients at the time of diagnosis (2).
The standard first-line treatment for ED-SCLC is the chemotherapy consisting of platinum (cisplatin or carboplatin) and etoposide (1). Most patients will show an initial response to chemotherapy, up to 40–70% of patients with ED-SCLC will achieve a complete response (CR) to first-line therapy (3). However, almost all ED-SCLC patients will experience a disease recurrence within the first year of therapy, with a median survival ranging from 8 to 13 months, the 5-year survival is only 1–2% (4). Unfortunately, second-line treatment options are currently limited for ED-SCLC patients, moreover, these patients often present resistance to second-line therapies. Limitations in current standard-of-care options and poor treatment outcomes have served as the impetus in the search for novel, alternative therapy approaches, including inhibition of vascular endothelial growth factor (VEGF), suppression of tumor angiogenesis, molecular targeted therapy and immunotherapy (5). Given the positive responses to immune checkpoint inhibitors in non-small-cell lung cancer (NSCLC), we have reason to theorize that similar responses will observed in SCLC (6).
The aim of cancer immunotherapy is to stimulate an immunoreaction to overcome the tumor avoidance of the immunologic surveillance system. Early phase clinical studies have identified several immune-checkpoint inhibitors, including pembrolizumab, nivolumab and ipilimumab, that show antitumor activity with durable responses in patients with SCLC (7,8). Patients who are associated with smoking and whose tumors have a high mutation burden are more likely to benefit from immunotherapy. Most patients with SCLC have these characteristics, therefore, exploring the clinical response to immune-checkpoint inhibitors in patients with SCLC is of clinical value (9).
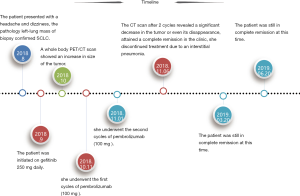
Here we report a case of an 86-year-old female patient with ED-SCLC. The patient was treated with pembrolizumab after experiencing the failure of first-line targeted therapy and achieved a CR. We present the following case in accordance with the CARE Reporting Checklist.
Case presentation
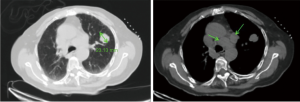
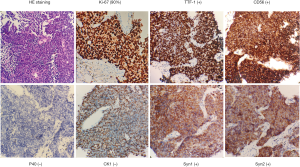
In August 2018, an 86-year-old female presented with a headache and dizziness. The patient had no specific personal or family history. A brain magnetic resonance imaging (MRI) scan was normal, but a computed tomography (CT) scan of the chest revealed a left-lung tumor with a 23.1 mm maximum diameter, as well as bilateral multiple mediastinal lymph node metastasis (Figure 1). She underwent a left-lung mass biopsy, and pathology confirmed SCLC (Figure 2). She was staged as ED-SCLC according to the Veterans Administration Lung Study Group. The patient was intolerant to first-line chemotherapy. Taking into account her advanced age and following the opinions of the patient and her families, the patient was initiated on gefitinib 250 mg daily from September 2018 without genetic test results. After a month of review, a whole body PET/CT scan showed an increase in size of the tumor.
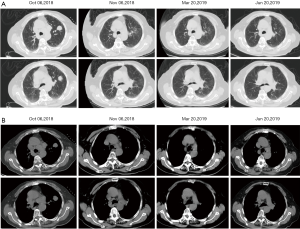
Given her intolerance to chemotherapy, the patient was thereafter received pembrolizumab intravenously at 100 mg every 3 weeks with a negative programmed death-ligand 1 (PD-L1) test result. From October 11, 2018 to November 01, 2018, she underwent 2 cycles of pembrolizumab, however, she discontinued treatment due to an interstitial pneumonia. What surprised us is that the CT scan after 2 cycles revealed a significant decrease in the tumor or even its disappearance, attained a complete remission in the clinic (Figure 3). The patient has continued to be supervised with CT scans and serum tumor marker measurements every 3 or 4 months and is still in complete remission at this time (Figure 4).
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
Immunotherapy has revolutionized the standard treatment across multiple tumor types (10-12). Immune checkpoints, which can inhibit immune responses and then contributed to immune escape, have been identified as negative regulatory factors in the process of tumor development. Two well-characterized checkpoints include the programmed cell death protein-1 (PD-1) and T-lymphocyte antigen-4 protein (CTLA-4) (13). Pembrolizumab is a highly selective, fully humanized monoclonal antibody that binds to the PD-1 receptor, blocking the interaction between PD-1 and its ligands, stimulating autoimmunity during T-cell activation, thereby preventing tumor avoidance of immunologic surveillance (11).
Pembrolizumab has shown promise as an agent with antitumor activity in patients with SCLC, consistent with its reported antitumor efficacy in other tumor types (10-13). The KEYNOTE-28 trial was a phase Ib multicohort study conducted to explore the safety and efficacy of pembrolizumab in ED-SCLC patients with PD-L1 positive tumors (12). Objective response rate (ORR), as the primary efficacy end point in the trial, was 33.3% and more favorable than the historical response rate for topotecan (range, 7% to 24%) (14). The tumor response was fast and lasting, with a median time to response of 2.0 months (range, 1.7 to 3.7 months) and a median duration of response of 19.4 months (range, ≥3.6 to ≥20.0 months). Notably, the tumor response time in our case was one month, and the patient still presented a state of complete remission from the initiation of immunotherapy until now. One possible explanation for this favorable therapeutic outcome in our patient was the high tumor mutational burden, which has been demonstrated as a predictive factor that could predict for clinical benefit with checkpoint inhibitors (15). Unfortunately, we lack direct data from our patient. The most common treatment-related adverse events (AEs) in the study were arthralgia, asthenia, and rash, as well as diarrhea and fatigue. In the present case, the reason for treatment discontinuation was not any of these above AEs but interstitial pneumonia.
Based on the KEYNOTE-28 trial, a large phase II study investigated the antitumor activity and safety of pembrolizumab in recurrent SCLC patients regardless of PD-L1 status was conducted. The results obtained in this KEYNOTE-158 study were consistent with those from the KEYNOTE-28 trial. The ORR was 18.7% (range, 11.8% to 27.4%) for the entire group and 35.7% (range, 24.0% to 84.0%) in PD-L1 positive patients (16). In June 2019, based on data from the KEYNOTE-28 and KEYNOTE-158 study, pembrolizumab, was granted approval by the US Food and Drug Administration (FDA) for treatment of metastatic SCLC patients who had made progress on a platinum-based chemotherapy and at least one other line of therapy. Although the current data from SCLC trials are limited, the above results suggest that pembrolizumab remains a promising addition to the cancer therapy armamentarium. Further researches which are evaluating the treatment of pembrolizumab to front-line, maintenance settings, and to combinations with chemotherapy, radiotherapy or other immune checkpoints are very necessary.
The PD-L1 level in tumor cells has been assessed as a predictive factor in several tumor types (13). However, our patient presented a negative PD-L1 test result and yet had a favorable therapeutic response. One possible explanation for this discordance is the low rate of PD-L1 positivity in SCLC patients. Yasuda et al. (17) assessed the expression of PD-L1 in 39 SCLCs and found only one patient with a positive test (≥1%), this finding was consistent with the study result (1 out of 30 patients) reported by Gadgeel et al. (9). Similarly, Bonanno et al. (18) found a lower expression of PD-L1 in SCLC patients with advanced stage disease than with early stage disease. The CheckMate 032 study reported only 18% of tumors with positive PD-L1 levels, however, both OS and PFS were similar to those reported in KEYNOTE 028 which only collected patients with PD-L1 positive tumors (12,19). Of note, a study indicated that SCLC patients with high expression levels of PD-L1 displayed a shorter survival (20). Given the evidences mentioned above, it seemed that there was no definite correlation between PD-L1 expression and the efficacy of immunotherapy in SCLC patients. Recent data have suggested that PD-L1 expression is more frequent in stromal cells than in SCLC tumor cells and that stromal cell expression may be a better predictive biomarker of efficacy (9). Therefore, the expression status of PD-L1 should not be the only measure condition of the application of immunotherapy.
Conclusions
In conclusion, pembrolizumab has demonstrated a promising antitumor activity in pretreated patients with ED-SCLC with resistance to other therapies, and offered a clinically meaningful therapeutic option. Further studies that move the treatment of pembrolizumab to front-line, maintenance settings and combinations with other treatment methods are necessary.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-590). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106-11. [Crossref] [PubMed]
- Stinchcombe TE. Current treatments for surgically resectable, limited-stage, and extensive-stage small cell lung cancer. Oncologist 2017;22:1510-7. [Crossref] [PubMed]
- Hagmann R, Hess V, Zippelius A, et al. Second-line therapy of small-cell lung cancer: topotecan compared to a combination treatment with adriamycin, cyclophosphamide and vincristine (ACO) - a single center experience. J Cancer 2015;6:1148-54. [Crossref] [PubMed]
- Chan BA, Coward JIG. Chemotherapy advances in small-cell lung cancer. J Thorac Dis 2013;5:S565-78. [PubMed]
- Cooper MR, Alrajhi AM, Durand CR. Role of Immune Checkpoint Inhibitors in Small Cell Lung Cancer. Am J Ther 2018;25:e349-56. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Ott PA, Bang YJ, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with Pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 2019;37:318-27. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II Study of Maintenance Pembrolizumab in Patients with Extensive-Stage Small Cell Lung Cancer (SCLC). J Thorac Oncol 2018;13:1393-9. [Crossref] [PubMed]
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1116-27. [Crossref] [PubMed]
- Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156-67. [Crossref] [PubMed]
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;35:3823-9. [Crossref] [PubMed]
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015;14:847-56. [Crossref] [PubMed]
- Asai N, Ohkuni Y, Kaneko N, et al. Relapsed small-cell lung cancer: treatment options and latest developments. Ther Adv Med Oncol 2014;6:69-82. [Crossref] [PubMed]
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018;33:853-61.e4. [Crossref] [PubMed]
- Chung HC, Lopez-Martin JA, Kao SCH, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol 2018;15:8506. [Crossref]
- Yasuda Y, Ozasa H, Kim YH. PD-L1 expression in small cell lung cancer. J Thorac Oncol 2018;13:e40-1. [Crossref] [PubMed]
- Bonanno L, Pavan A, Dieci MV, et al. The role of immune microenvironment in small-cell lung cancer: Distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur J Cancer 2018;101:191-200. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicenter randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Xu Y, Cui G, Jiang Z, et al. Survival analysis with regard to PD-L1 and CD155 expression in human small cell lung cancer and a comparison with associated receptors. Oncol Lett 2019;17:2960-8. [PubMed]