
Effect of perfluorocarbon partial liquid ventilation—induced hypothermia on dogs with acute lung injury
Introduction
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS), first described by Ashbaugh in 1967 (1), is defined as diffuse alveolar-capillary membrane damage due to severe infection, trauma, shock, or acidosis. It involves intrapulmonary and external pathogenic factors and is characterized by progressive respiratory failure and refractory hypoxemia (2). This is a particularly common disease with a high incidence rate and mortality in critically ill patients (3,4). The conventional treatment in clinical practice for this common emergency disease for cases with serious condition and poor prognosis is based on mechanical ventilation. The treatment of ALI/ARDS can reduce the accumulation of pulmonary edema by lung protective ventilation, that is, by retaining the barrier properties of alveolar endothelium and alveolar epithelium. It can also be used to treat ALI/ARDS with fluid conservative method, that is to reduce the hydrostatic pressure of pulmonary vessels, reduce pulmonary edema and increase pulmonary vascular permeability (5). Although previous studies have increased our understanding of the molecular mechanism of ALI/ARDS, an ideal treatment is still lacking (6).
Partial liquid ventilation (PLV) is a kind of ventilation method which has been developed in recent years. Perfluorocarbon (PFC) is a transparent liquid composed of carbon and fluorine without color and odor at normal temperature; it has good tissue compatibility and no absorption or metabolism in the body, which thus makes it an ideal liquid venting medium. There is an abundance of studies confirming that PFC-mediated PLV can significantly improve the gas exchange function and respiratory dynamics of ALI (7-9). Low temperature provides an organ-protective effect and can reduce the expression of inflammatory factors in ALI (10). The lungs are not only a place for gas-blood exchange, but also a place for heat exchange, and thus a good cooling effect can be achieved through the lungs (11,12).
In this study, low-temperature fluorocarbon partial fluid ventilation was used to induce shallow hypothermia in dogs with ALI to study the influence of fluorocarbon partial fluid ventilation combined with mild hypothermia including ALI/ARDS canine hemodynamics, lung function, inflammatory factors in peripheral blood and lung tissue, and the expression level of NF-κB p65.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1275).
Methods
Animals
This study was approved by the ethics committee of the First Affiliated Hospital of Nanchang University (No. 2018-053). All animals in the study were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University, following the guidelines for the protection and use of laboratory animals in Nanchang University. A total of 36 healthy male mixed-bred dogs weighing 9.5 to 11.8 kg, with an average of 10.15±0.89 kg, were purchased from the Animal Department of Nanchang University Medical College and kept in a normal environment.
ALI model
Oleic acid-induced ALI animal models were induced according to the method described by Nakazawa et al. (13). First, animals were weighed and intramuscularly injected with ketamine hydrochloride 10 mg/kg (batch number: KH140408, Jiangsu Hengrui Pharmaceutical Co., Ltd., China) and midazolam 0.4 mg/kg (batch number: 20160609, Jiangsu Enhua Pharmaceutical Co., Ltd., China) for general anesthesia. The dogs were placed in the supine position on the experimental bench, intubated with an ID 7.0 mm endotracheal tube, and mechanically ventilated in a ventilator (PB840 ventilator, Puritan-Bennett, USA). During the experiment, intravenous midazolam 0.2–0.3 mg/kg·h and vecuronium 0.1–0.2 mg/kg·h (Yangtze River Pharmaceutical Group Co., Ltd., China) were used to maintain anesthesia and muscle relaxants, and accompanied by intravenous infusion of Ringer’s lactate solution 5–10 mL/kg·h. The electrocardiogram (ECG) was continuously monitored on the left and right forelimbs of the animals and the intimate electrodes of the left hind limb (MP20 multi-function monitor, Philips, the Netherlands). The internal jugular vein was separated, and a Swan-Ganz catheter (Edward) was inserted into the pulmonary artery through the internal jugular vein. The room temperature was maintained at 22 °C [(22±0.5) °C].
High purity 95% oleic acid (01008, SIGMA, USA) was uniformly injected 3 times at 0.10–0.15 mL/kg through the right jugular vein for 10 min with a sampler. The blood gas was analyzed every 10 min after the airway pressure started to increase. Detection of arterial blood gas was repeated until the oxygenation index (PaO2/FiO2) ≤200 mmHg (1 mmHg =0.133 kpa). The preparation of the ALI model was considered successful if this could be maintained for 30 min. If the blood gas could not reach the above standard after 1 h, more oleic acid was injected at 0.02 mL/kg. The average modeling time was 1.6±0.6 h. The ALI model was successfully prepared in 24 experimental dogs. When anesthesia was stable for 0.5 h, this time-point was defined as T0, and the successful ALI modeling was defined as T1. Times at 1, 2, 3, and 4 h after ALI modeling thereafter were defined as T1, T2, T3, and T4, respectively.
Experimental grouping and treatment
All animals were divided into three groups with nine animals in each group according to the random number table method. Description of the groups follow below.
- Conventional mechanical ventilation group (CMV group): mechanical ventilation with a ventilator was applied after successful ALI modeling. The respiratory parameters were as follows: tidal volume (VT), 8 mL/kg; respiratory rate (f), 20 bpm; oxygen concentration (FiO2), 0.8; inspiratory to expiratory ratio (I:E), 1:2; positive end-expiratory pressure (PEEP), 5 cmH2O (1 cmH2O =0.098 kpa). The experimental bench was covered with heating blanket. The dog’s anus temperature was maintained at 36.5–37.5 °C.
- Normal temperature PFC liquid ventilation group (NPLV group): after the dog ALI model was successfully developed, mechanical ventilation was given and the PFC at 37 °C normal temperature was slowly injected into the lung through the side hole of the tracheal intubation at 10–15 mL/kg. The ventilator parameter settings and temperature were set to be identical to the CMV group.
- Hypothermic PFC liquid ventilation group (HPLV group): after successful ALI modeling, the dog was given mechanical ventilation, and a 15 °C low-temperature PFC PLV was performed through the tracheal tube with PFC at 10–15 mL/kg to reduce the core temperature of the dog: the rectal temperature was 33–36 °C. This temperature was then maintained with 32 °C PFC ventilation. The ventilator parameters were set to be identical to the CMV group. An electronic temperature probe was placed 6 cm from the anus to monitor the rectal temperature.
Pulmonary function assessment
Between T0 and T5, arterial blood was sampled under the following conditions: VT, 8 mL/kg; respiratory frequency (f), 20 bpm; PEEP, 5 cmH2O. The blood gas was analyzed by ABL720 blood gas analyzer (GMI, USA), and the oxygenation index (PaO2 ÷ FiO2) was calculated. Plateau pressure (Pplat), peak airway pressure (PIP), and average airway resistance (RAW) were monitored and recorded. Lung static compliance (CLst) was calculated as follows: Cstat = VT/(Pplat-total PEEP). A pressure sensor was connected to the multi-function monitor, and the pulmonary function meter was connected to the ventilator and endotracheal tube catheter interface.
Pulmonary edema assessment
Lung edema was evaluated by lung wet/dry weight ratio (W/D). The right middle lobe tissues were collected from experimental animals, rinsed with normal saline, dried with filter paper, and weighed for measurements of wet weight (W) with electronic analyzer. Next, the tissues were dried at 80 °C for 48 h to obtain the dry weight (D). The W/D was then calculated.
Hemodynamics
Cardiac output (CO) was measured between T0 and T5 by the Swan-Ganz catheter. When measuring CO, 10 mL of ice-cold saline (4 °C) was injected within 4 s. The measurement was repeated three times, the average value was calculated, and heparinized anticoagulation was performed. Heart rate (HR) was monitored with a multifunctional monitor. The mean arterial pressure (MAP) was detected by a pressure sensor connected to the multi-function monitor.
Enzyme-linked immunosorbent assay (ELISA)
For the ELISA 5 mL of peripheral venous blood was taken from T0 to T5. The serum was centrifuged at 2,000 r/min for 10 min, and then stored in a refrigerator at −80 °C. At T5, the animals were sacrificed by excessive anesthesia, the thoracic cavity was quickly opened, and the right lung was freed and ligated. The left lung was lavaged with bronchoalveolar lavage 3 times with normal saline at 5 mL/kg, and bronchoalveolar lavage fluid (BALF) was collected and centrifuged. The supernatant was stored at −20 °C. ELISA kits (Bluegene, China) were used to measure the concentrations of IL-10 (Abcam, Cambridge, UK) and TNF-α (Abcam, Cambridge, UK) in serum and BALF, separately.
Western blotting assay
The expression level of NF-κB p65 was detected by Western blotting assay. The right lower lobe tissues were homogenized in cold radioimmunoprecipitation (RIPA) lysis buffer (Sigma) containing protease inhibitors. Determination of protein concentration in tissues were conducted by bicinchoninic acid assay kits (Beyotime Biotech Co., China). After centrifugation, proteins were separated by 10% SDS-PAGE gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Burlington, MA, USA). The membrane was then sealed with 5% skimmed milk for 2 h and incubated overnight at 4 °C with rabbit anti-NF-κB p65 antibody (1:1,000, cell signaling technology, Beverly, MA, USA). After rinsing the membrane three times, the membrane was incubated with the rabbit anti-goat secondary antibody (1:1,000; cell signaling technology) at 25 °C for 1 h. The protein bands were visualized and quantified by gel imaging system (Thermo Fisher Scientific) and Image-Pro-Plus software respectively. β-actin was used as internal reference antibody. Both the first and secondary antibodies were purchased from Cell Signaling Technology Inc.
Statistical analysis
The SPSS21.0 statistical software package was used for data processing. The measurement data were presented as standard deviation (SD). The comparison between groups was performed by independent sample Student’s t-test. Analysis of variance (ANOVA) was used to compare repeated measurement at different time points in the group. The difference was considered as statistically significant at P<0.05.
Results
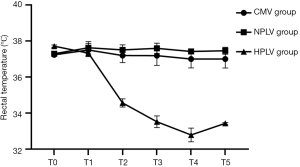
Changes of rectal temperature
After Ali model was established and different ventilation was carried out, rectal temperature of each group was measured. Figure 1 shows the changes of rectal temperature across the three groups. The rectal temperature of the dogs in the HPLV group was significantly lower than that in the NPLV and CMV groups from T1 to T4 (P<0.05). The results showed that low temperature perfluorocarbon liquid ventilation could decrease the rectal temperature continuously.
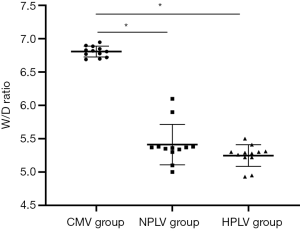
Effects of hypothermic PLV on pulmonary edema in the ALI model
For the effect of fluorocarbon PLV at different temperatures on pulmonary edema in ALI dogs, we evaluated pulmonary edema by W/D index. Figure 2 shows that, compared with the CMV group, the W/D was significantly decreased in the HPLV group and the NPLV group (P<0.05). The results show that perfluorocarbon liquid ventilation can relieve pulmonary edema in dogs.
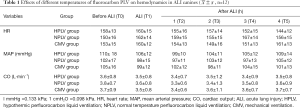
Hemodynamic changes
In order to explore the hemodynamic stability of experimental dogs after different ventilations, we tested the heart rate (HR), MAP, and CO of three groups of dogs. There was no significant difference in the heart rate (HR), MAP, and CO between the HPLV group, NPLV group, and CMV group at the T0 to T5 time-points (P>0.05). This suggests that the hemodynamics of canines in the three experimental groups were stable (Table 1).
Full table
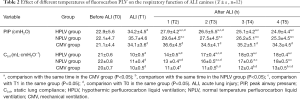
Effects of hypothermic PLV on pulmonary functions in the ALI model
Compared with T0, the airway peak pressure (PIP) and lung compliance (CLst) were significantly increased at each time-point (T1–T5) in the HPLV group, NPLV group, and CMV group (P<0.05) (Table 2). Table 2 also shows that the PIP was significantly decreased and the CLst was significantly increased in the HPLV group and the NPLV group between the T2 and T5 time-points, as compared with the CMV group (P<0.05).
Full table
The PaO2 and PaO2/FiO2 levels of each experimental group were significantly decreased after the ALI model was successfully established (P<0.05) (Table 3). Compared with the CMV group, the PH value of the HPLV group and the NPLV group was significantly increased at the T4 and T5 time points (P<0.05) (Table 3). PaO2 and PaO2/FiO2 in the HPLV group and the NPLV group were significantly increased from the T2 to T5 time-points as compared to the CMV group (P<0.05) (Table 3). This suggests that PLV could significantly improve the gas exchange function and respiratory dynamics of ALI canines. Compared with the NPLV group, the PaO2 and PaO2/FiO2 values in the HPLV group were significantly increased at the T4 andT5 time-points (P<0.05), indicating that hypothermic PLV could supply better oxygenation (Table 3).

Full table
The levels of IL-10 and TNF-α in peripheral blood serum
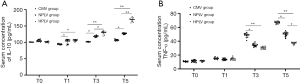
IL-10 is an anti-inflammatory factor. As shown in Figure 3A, the levels of IL-10 in the HPLV group and the NPLV group were significantly higher than those in the CMV group in the T2–T5 time-points. In addition, serum IL-10 in the HPLV group increased significantly at the T3 and T5 time-points compared with the NPLV group. TNF-α is a proinflammatory factor. Compared with the CMV group, the concentrations of serum TNF-α in the HPLV group and NPLV group were significantly decreased (Figure 3B). The TNF-α level in the HPLV group at the T5 time-point was significantly lower than that in the NPLV group (Figure 3B). These results suggested that low-temperature PLV could affect the concentration of IL-10 and TNF-α, and alleviate the inflammatory injury of ALI in serum.
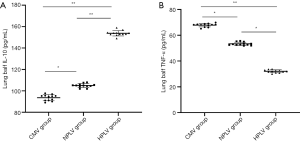
The levels of IL-10 and TNF-α in BALF of the ALI model
The concentration of IL-10 in the HPLV group and NPLV group was significantly higher than that of the CMV group in BALF (Figure 4A). Compared with the NPLV group, the IL-10 level was significantly increased in the BALF of the HPLV group (Figure 4A). Figure 4B shows that TNF-α was significantly decreased in the BALF of the HPLV group and the NPLV group, when compared with the CMV group. Moreover, the TNF-α level of the NPLV group was significantly lower than that of the HPLV group in BALF (Table 4). The results indicate that low-temperature PLV could regulate IL-10 and TNF-α levels, and alleviate the inflammatory injury of ALI in BALF.
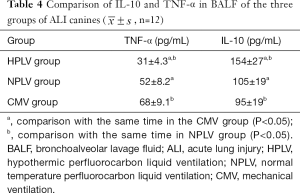
Full table
Western blotting assay in BALF
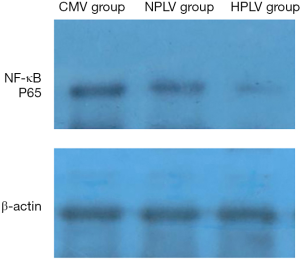
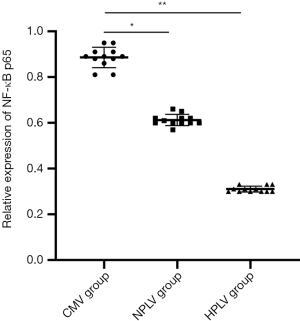
The immunoblots of NF-κB p65 in the BALF of the CMV group, the HPLV group, and the NPLV group are shown in Figure 5. Compared with the CMV group, the expression levels of NF-κB p65 were significantly decreased in the BALF of the HPLV group and the NPLV group. The expression levels of NF-κB p65 in the BALF of the NPLV group were significantly lower than those of the HPLV group (Figure 6). These results suggest that hypothermic PLV could downregulate the expression of NF-κB p65 in BALF.
Discussion
ALI/ARDS is a syndrome characterized by diffuse alveolar inflammation and alveolar interstitial edema. Due to hypoxemia from multiple causes, the mortality rate of ARDS is as high as 30–50% (14). PFC has the ability to dissolve a large amount of gas, including O2 and CO2. Moreover, PFC features rapid release, low surface tension, high specific gravity, good volatility, good tissue compatibility, and no absorption or metabolism in the body, which makes PFC an ideal liquid venting medium. However, Low temperature can reduce the overexpression and release of cytokines in lung tissue, inhibit neutrophil aggregation, and reduce oxygen consumption in lung tissue (15,16). The lung is composed of about 5×108 alveoli with a surface area of about 100 m2. Alveoli are rich in capillaries, the pulmonary capillary surface is about 70 m2, and the alveolar thickness is about 0.5–10 µm. These physiological characteristics of the lung suggest that the cooling of the lung is feasible (17-19). Our results confirmed that the use of cold fluorocarbon PLV can quickly reduce body temperature: 15 °C fluorocarbon treatment for 15–30 min reduced the rectal temperature to 33–35 °C, after which shallow hypothermia could be maintained at 32 °C with PFC PLV.
It is unclear whether a sudden reduction in bronchial and pulmonary temperature affects hemodynamics (20,21) or respiratory mechanics, especially airway resistance (22). Therefore, the purpose of this study was to evaluate the effects of transpulmonary systemic temperature reduction on systemic hemodynamics, respiratory mechanics, blood gas analysis, and inflammatory factors.
Intravenous oleic acid induces endothelial destruction, and pulmonary vascular permeability increases immediately, resulting in the accumulation of extravascular fluid in the lungs (23). We found that after oleic acid infusion the static compliance of CLst was significantly reduced, and the peak pressure of the airway increased; meanwhile, the gas exchange was generally impaired, PaO2 and PaO2/FiO2 decreased significantly, and PaCO2 gradually increased. We found that the NPLV and HPLV group (T2–T5 time points) had significantly improved lung static compliance CLst after fluorocarbon PLV, and the PIP gradually decreased. Compared with the CMV group, PaO2 and PaO2/FiO2 in the NPLV and HPLV groups increased significantly, and there was no significant difference in CLst and PIP between the HPLV group and the NPLV group at different time-points. PFC plays a gas exchange role in the lungs and establishes a PEEP to stabilize the alveolar structure (24), reopen the collapsed alveoli, improve the ventilation/blood flow ratio, and increase lung compliance. Moreover, PFC has an excellent ability to carry oxygen and carbon dioxide, increase oxygen concentration in unit volume of alveoli, improve lung gas exchange function, raise PaO2 (25), decrease carbon dioxide accumulation, and reduce acidosis (26).
Our results showed that PaO2 and PaO2/FiO2 levels in the HPLV group were significantly higher than those in the NPLV group at 3 to 4 h after ALI, and the W/D ratio of lung tissue in the HPLV group was decreased. It may be that low-temperature PFC PLV reduces bronchial temperature and systemic temperature, slows body metabolism, decreases oxygen consumption, and is more conducive to improving the oxygenation function of ALI dogs. Moreover, compared with normal temperature PLV, low-temperature fluorocarbon PLV does not cause significant changes in hemodynamics and pulmonary respiratory mechanics.
It is generally believed that ARDS is caused by the imbalance of inflammatory mediators and anti-inflammatory mediators in the lungs, which leads to excessive and uncontrolled inflammatory reactions and finally results in a waterfall-like inflammatory injury and secondary diffuse lung parenchymal injury (27). The animal model of oleic acid-induced lung injury better simulates the pathophysiological manifestations of patients with ARDS caused by severe trauma, multiple fractures, and fat embolism. An ARDS model independent of inflammatory cells and their active products can help us to understand the role of inflammatory cells and their active products in the pathogenesis of ARDS. TNF-a and IL-6 are pro-inflammatory cytokines that mediate neutrophil (PMN) activation and adhesion to vascular endothelial cells. The accumulation of a large amount of chemokines at inflammation sites causes degranulation of PMN and release of lysosomal enzymes, which results in the damage of alveolar epithelial cells or endothelial cells, and consequently increases the permeability of alveolar capillary membranes and aggravates lung injury. Hou et al. (28) conducted a study on lipopolysaccharide-induced lung injury, which showed that PFC can prevent PMN from infiltrating lung tissue and alleviate pulmonary edema. Furthermore, Gale et al. (29) found that PFC inhibits the activity of PMN in rats, thereby inhibiting the production and release of inflammatory factors by macrophages. Our results showed that serum IL-10 was significantly elevated in the HPLV group 4 h after low-temperature fluorocarbon liquid ventilation, and serum TNF-α concentration was significantly lower than that in the NPLV group and the CMV group. Compared with the NPLV group, HPLV group had decreased TNF-α and increased IL-10 levels in the BALF of the lung tissue and the expression of NF-κB p65 in the lung tissue was downregulated, suggesting that combining PFC PLV with shallow hypothermia may increase anti-inflammatory responses and inhibit the production and release of pro-inflammatory factors. In recent years, some studies have shown that shallow hypothermia treatment can reduce ventilator-induced lung injury in animal models of ALI by reducing the release of pro-inflammatory cytokines, TNF-α and IL-6, in lung tissue and plasma (30,31), increasing the amount of anti-inflammatory cytokines (IL-10) in lung tissue (32,33), and improving pulmonary vascular manifestations and alveolar epithelial damage (34).
The limitations of our study include the relatively short treatment time with at the low temperature. Prolonged low temperature PLV time may produce more findings. In addition, we chose mixed-breed dogs for our model due to the experimental conditions, which might have resulted in a standard error.
In summary, the use of low-temperature fluorocarbon partial fluid ventilation to reduce systemic temperature to 33–35 °C can improve respiratory function, reduce ALI-induced inflammatory factors, and increase anti-inflammatory response.
Acknowledgments
Funding: This study was supported by the Science and Technology Planning Project of Jiangxi Science and Technology Department (No. 20151BBG70209).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1275
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1275
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1275). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of the First Affiliated Hospital of Nanchang University (No. 2018-053). All animals in the study were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University, following the guidelines for the protection and use of laboratory animals in Nanchang University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Spinelli E, Grieco DL, Mauri T. A personalized approach to the acute respiratory distress syndrome: recent advances and future challenges. J Thorac Dis 2019;11:5619-25. [Crossref] [PubMed]
- Yehya N. Lessons learned in acute respiratory distress syndrome from the animal laboratory. Ann Transl Med 2019;7:503. [Crossref] [PubMed]
- Rajasekaran S, Pattarayan D, Rajaguru P, et al. MicroRNA Regulation of Acute Lung Injury and Acute Respiratory Distress Syndrome. J Cell Physiol 2016;231:2097-106. [Crossref] [PubMed]
- Estenssoro E, Dubin A. Acute respiratory distress syndrome. Medicina (B Aires) 2016;76:235-41. [PubMed]
- Laffey JG, Kavanagh BP. Fifty Years of Research in ARDS. Insight into Acute Respiratory Distress Syndrome. From Models to Patients. Am J Respir Crit Care Med 2017;196:18-28. [Crossref] [PubMed]
- Diep BA, Chan L, Tattevin P, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 2010;107:5587-92. [Crossref] [PubMed]
- Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neuro-logical status among adults with cardiac arrest: a randomized clinical trial. JAMA 2014;311:45-52. [Crossref] [PubMed]
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197-206. [Crossref] [PubMed]
- Kimura A, Sakurada S, Ohkuni H, et al. Moderate hypothermia delays proinflammatory cytokine production of human peripheral blood mononuclear cells. Crit Care Med 2002;30:1499-502. [Crossref] [PubMed]
- Kumar MM, Goldberg AD, Kashiouris M, et al. Transpulmonary hypothermia: a novel method of rapid brain cooling through augmented heat extraction from the lungs. Resuscitation 2014;85:1405-10. [Crossref] [PubMed]
- Sage M, Nadeau M, Kohlhauer M, et al. Effect of ultra-fast mild hypothermia using total liquid ventilation on hemodynamics and respiratory mechanics. Cryobiology 2016;73:99-101. [Crossref] [PubMed]
- Nakazawa K, Yokoyama K, Matsuzawa Y, et al. Pulmonary administration of prostacyclin (PGI2) during partial liquid ventilation in an oleic acid-induced lung injury: inhalation of aerosol or intratracheal instillation? Intensive Care Med 2001;27:243-50. [Crossref] [PubMed]
- Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome:incidence and mortality, has it changed? Curr Opin Crit Care 2014;20:3-9. [Crossref] [PubMed]
- Aibiki M, Maekawa S, Ogura S, et al. Effect of Moderate Hypothermia on Systemic and Internal Jugular Plasma IL-6 Levels after T raumatic Brain Injury in Human . J Neurotrauma 1999;16:225-32. [Crossref] [PubMed]
- Kira S, Daa K. Mild Hypothermia Reduces Ex pression of Intercellular Adhesion M olecule-1 (ICAM-1) and the Accumulation of Neutrophils after Acid- induced Lung Injury in the Rat. Acta Anaesthesiol Scand 2005;49:351-9. [Crossref] [PubMed]
- Harris SB, Darwin MG, Russell SR, et al. Rapid (0.5 degrees C/min) minimally invasive induction of hypothermia using cold perfluorochemical lung lavage in dogs. Resuscitation 2001;50:189-204. [Crossref] [PubMed]
- Kumar MM, Goldberg AD, Kashiouris M, et al. Transpulmonary hypothermia: a novel method of rapid brain cooling through augmented heat extraction from the lungs. Resuscitation 2014;85:1405-10. [Crossref] [PubMed]
- Sage M, Nadeau M, Kohlhauer M, et al. Effect of ultra-fast mild hypothermia using total liquid ventilation on hemodynamics and respiratory mechanics. Cryobiology 2016;73:99-101. [Crossref] [PubMed]
- Gordon C, Collard CD, Pan W. Intraoperative management of pulmonary hypertension and associated right heart failure. Curr Opin Anaesthesiol 2010;23:49-56. [Crossref] [PubMed]
- Kerans V, Espinoza A, Skulstad H, et al. Systolic left ventricular function is preserved during therapeutic hypothermia,also during increases in heart rate with impaired diastolic filling. Intensive Care Med Exp 2015;3:41. [Crossref] [PubMed]
- Koskela HO. Cold air-provoked respiratory symptoms:the mechanisms and management. Int J Circumpolar Health 2007;66:91-100. [Crossref] [PubMed]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379-9. [Crossref] [PubMed]
- Forgiarini LA Jr, Forgiarini LF, da Rosa DP, et al. Endobronchial perfluorocarbon administration decreases lung injury in an experimental model of ischemia and reperfusion. J Surg Res 2013;183:835-40. [Crossref] [PubMed]
- Wang X, Zhang J, Li X, et al. Sustained improvement of gas exchange and lung mechanics by vaporized perfluorocarbon inhalation in piglet acute lung injury model. Clin Respir J 2014;8:160-6. [Crossref] [PubMed]
- Cho JS, Kim YD, Shin N, et al. Effect of transpleural perfusion with oxygenated perfluorocarbon in a rat model of acute lung injury. Exp Lung Res 2013;39:32-8. [Crossref] [PubMed]
- Thompson BT, Matthay MA. The Berlin definition of ARDS versus pathological evidence of diffuse alveolar damage. Am J Respir Crit Care Med 2013;187:675-7. [Crossref] [PubMed]
- Hou S, Ding H, Lv Q, et al. Therapeutic effect of intravenous infusion of perfluorocarbon emulsion on LPS-induced acute lung injury in rats. PLoS One 2014;9:e87826. [Crossref] [PubMed]
- Gale SC, Gorman GD, Copeland JG, et al. Perflubron emulsion prevents PMN activation and improves myocardial functional recovery after cold ischemia and reperfusion. J Surg Res 2007;138:135-40. [Crossref] [PubMed]
- Hong SB, Koh Y, Lee IC, et al. Induced hypothermia as a new approach to lung rest for the acutely injured lung. Crit Care Med 2005;33:2049-55. [Crossref] [PubMed]
- Lim CM, Kim EK, Koh Y, et al. Hypothermia inhibits cytokine release of alveolar macrophage and activation of nuclear factor kappaB in endotoxemic lung. Intensive Care Med 2004;30:1638-44. [Crossref] [PubMed]
- Cruces P, Erranz B, Donoso A, et al. Mild hypothermia increases pulmonary anti-inflammatory response during protective mechanical ventilation in a piglet model of acute lung injury. Paediatr Anaesth 2013;23:1069-77. [Crossref] [PubMed]
- Lim CM, Hong SB, Koh Y, et al. Hypothermia attenuates vascular manifestations of ventilator-induced lung injury in rats. Lung 2003;181:23-34. [Crossref] [PubMed]
- Huet O, Kinirons B, Dupic L, et al. Induced mild hypothermia reduces mortality during acute inflammation in rats. Acta Anaesthesiol Scand 2007;51:1211-6. [Crossref] [PubMed]
(English Language Editor: J. Gray)


