
An esophageal cancer case of cytokine release syndrome with multiple-organ injury induced by an anti-PD-1 drug: a case report
Introduction
In the tumor microenvironment, tumor cells escape immune recognition and responses by over-expressing various immunosuppressive molecules (1). The anti-PD-1 drug is a kind of immune-regulator, which has been used to treat various types of tumors by blocking the negative regulatory signal of T cells to relieve the immunosuppression reactions (2,3). However, immune hyperactivation induced by anti-PD-1 drugs could cause cytokine release syndrome (CRS) (4). CRS refers to a violent cytokine release (including TNF-α, IL-1, IL-6, IL-12, IFN-α, IFN-β, IFN-γ, MCP-1, and IL-8) caused by infection or immune disorders, and it is a significant cause of acute respiratory distress syndrome and multiple organ failure. When anti-PD-1 drugs target healthy tissues, CRS is a commonly observed phenomenon, and it usually leads to immune-related adverse events (irAEs). Furthermore, radiotherapy may also elicit severe CRS (5). Usually, irAEs include fever, hypotension, heart problems, dyspnea, tiredness, nausea, and coagulation disorders (5-8). irAEs can also damage many tissues and organs, including skin, digestive tract, liver, endocrine, lung (9). Hematological toxicities (0.5%) (10) and renal immune-related adverse reactions (1%) (11,12) were relatively rare; these events recorded were often clinically severe and life-threatening (13,14). An increased number of reports on anti-PD-1 drugs induced CRS (15,16), or irAEs (17-23), has been observed in recent years.
In this report, we for the first time reported an elderly esophageal cancer (ESC) case of CRS and irAEs induced by radiotherapy following anti-PD-1 drug treatment, including diarrhea, immune thrombocytopenia, and multiple-organ injuries.
We present this case following the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1310).
Case presentation
A 69-year-old male patient with ESC was hospitalized on December 2, 2019, after three cycles of chemotherapy with docetaxel and nedaplatin accompanied by the addition of PD-1 inhibitor (Sintilimab). The chemotherapy scheme was interrupted due to the absence of any response. Instead, three-dimensional conformal radiotherapy (60 Gy/30 times, 5 times/week) was locally applied to treat the esophageal lesion. After 6 times of radiotherapy, the typical CRS and irAEs occurred, which were highly possibly induced by radiotherapy following the PD-1 inhibitor, including fever, diarrhea, hematological inflammation, renal damage, and so on. After the combination treatment of glucocorticoids and other immunomodulators, the irAEs symptoms were improved, and the patient get discharged from the hospital. The fundamental courses are as follows.
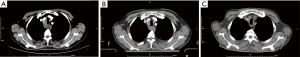
In July 2019, the patient was first referred to the hospital due to the main symptom of dysphagia for more than one month and was diagnosed as local advanced squamous cell carcinoma of the upper esophagus (mediastinal lymph node metastasis and close relationship with blood vessels and trachea), which was unsuitable for surgery upon the diagnosis (Figure 1A). Then, the patient was given neoadjuvant chemotherapy with docetaxel and nedaplatin (28 days/cycle), as well as anti-PD-1 (Sintilimab, 3 weeks/cycle) immunotherapy simultaneously. The process of treatment was smooth without significant side reactions.
On August 17, 2019, and September 26, 2019, the patient was admitted to the hospital, and given the second and third cycle of chemotherapy (docetaxel + nedaplatin) scheme with a PD-1 inhibitor (Sintilimab), the process was smooth without significant side reactions.
At the end of October2019, the patient suffered from diarrhea and fever, which delayed the chemotherapy. So, the patient was admitted to the hospital for only PD-1inhibitor (Sintilimab) treatment on November 7, 2019.
On December 2, 2019, the patient was admitted to the hospital for the fourth chemotherapy. However, the patient suffered from intermittent cough, expectoration, and diarrhea on December 7, 2019. The imaging results showed inflammatory lung lesions, which were considered the adverse events of the anti-PD-1 treatment. After glucocorticoid treatment, symptoms improved. However, the CT scan on December 10, 2019 (Figure 1B), indicated the disease had no response to the chemotherapy, and it was unsuitable for routine surgery and chemotherapy.
Then, the three-dimensional conformal radiotherapy was locally applied to treat the esophageal lesion (60 Gy/30 times, 5 times/week). The patient suffered from mild diarrhea, intermittent low fever, and leukopenia after 6 times of radiotherapy, and the symptoms were improved after the symptomatic treatment with hormone, antidiarrheal, and other medicines. The platelet level was significantly decreased to 31×109/L, and the D-dimer level was significantly elevated to 7.023 mg/L after 10 times of radiotherapy. Then, radiotherapy was stopped, and the patients received symptomatic treatment. CT showed that scattered inflammation in both lung, that mediastinal lymph nodes and esophageal lesions were significantly reduced (Figure 1C), and the platelet count dropped to the lowest level of 18×109/L 2 days later. The following conditions were considered: (I) disseminated intravascular coagulation; (II) side effects of the PD-1 inhibitor; (III) bone marrow metastasis. Then, the patient received a bone marrow smear and symptomatic platelet supplement therapy. Bone marrow smears showed that megakaryocyte maturation was blocked. After 4 days of symptomatic treatment with platelet supplement, the blood system did not improve significantly. The levels of IL-6, IL-10 and IL-17A cytokines in peripheral blood were significantly increased. It can be inferred that the irAEs was caused by CRS or PD-1 inhibitors, considering thrombocytopenia after two months of anti-PD-1 drugs. Meanwhile, the patient showed diarrhea, tremor, hypocalcemia, and hyperkalemia, and the serum creatinine of the patients sharply increased from 78.4 to 609.5 µmol/L in 24 h.
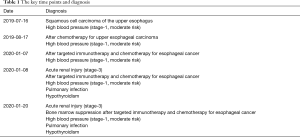
Moreover, the thyroglobulin (TG) and thyroid microsome (TM) antibodies significantly increase (62.4 and 24.43 IU/mL),which implies that Hashimoto’s thyroiditis might be triggered as an autoimmune inflammatory response. We presumed there might be multisystem injuries (including nervous system injury, thyroid injury, and acute renal injury) caused by CRS after the application of the anti-PD-1 drug. Then, the diagnosis of this ESC patient was modified as acute renal injury (Stage-3), after targeted immunotherapy and chemotherapy for ESC, high blood pressure (Stage-1, moderate risk), pulmonary infection, and hypothyroidism. These were obvious CRS and irAEs involved in multiple organs (including blood, kidney, and thyroid). Hormone impact treatment (methylprednisolone, 80 mg) was given in combination with intravenous injection of recombinant human anti-IL-6 receptor monoclonal antibody tocilizumab (80 mg), mycophenolate mofetil (oral bid, 1 g), levothyroxine sodium tablets (oral QD, 25 µg) and human immunoglobulin (intravenous injection, 20 g). After 12 days of treatment, the patient showed no symptom of fever, cough, and expectoration. The platelet count returned to the normal level (125×109/L). The serum creatinine level fell to 170.8 µmol/L, indicating that the hematological inflammation and renal damage were improved. The key time points and diagnoses are presented in Tables 1 and 2.
Full table

Full table
On discharge from hospital, the general situation was as follows: no fever, no cough, no expectoration, no diarrhea, no tremor, 4,790 mL urine volume. The temperature was normal (36.5 °C), the platelet count was 125×109/L, the serum creatinine level was 170.8 µmol/L, the procalcitonin and C-reactive protein levels were 0.083 ng/mL and 2.93 mg/mL, respectively. The discharge diagnosis was as follows: acute renal injury (Stage-3), bone marrow suppression after targeted immunotherapy and chemotherapy for ESC, high blood pressure (Stage-1, moderate risk), pulmonary infection, and hypothyroidism. The patient was given the following discharge orders: (I) the patient should take sufficient rest and increase proper intake of calories and nutrients, (II) methylprednisolone 40 mg should be taken orally in the morning combined with 0.6 g vitamin D calcium, (III) mycophenolate mofetil (1 g) should be taken orally twice a day, (IV) levothyroxine sodium tablets 25 µg orally once a day, (V) one day after discharge, blood routine examination, coagulation function, and electrolyte should be tested; and three days after discharge, liver and kidney function, myocardial enzyme and thyroid function should be conducted, (VI) all drugs need to be adjusted according to follow-up results, (VII) the patient should be treated regularly in the oncology department for ESC, (VIII) this patient should be strictly followed up for 5 years.
Written informed consent was obtained from the patient. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Discussion
Anti-PD-1 drug-related side effects are nonspecific reactions caused by activating the immune system, which can affect almost all tissues and organs (9). We first described the typical CRS and irAEs case with multiple-organ injuries during radiotherapy following anti-PD-1 drug treatment.
CRS is a severe over-immune response. After the treatment of chimeric antigen receptor-modified T-cell (CAR-T) or bispecific T-cell receptor engagement immunology, T cells are activated and proliferate rapidly, resulting in excessive cascade release of cytokines. The positive feedback cycle causes it between cytokines and immune cells (5,6). These cytokines mediate various immune responses, resulting in fever, coagulation disorders, and multiple organ failure (5-8). At present, there are few reports about CRS induced by anti-PD-1 drugs (15,16). In 2017, a case with alveolar soft tissue sarcoma was reported by Rotz et al. (5). This patient developed severe CRS with hyperthermia, encephalopathy, hypotension, anoxia, liver dysfunction, and coagulation disorder after anti-PD-1 treatment. The patient was relieved after the infusion of the IL-6 inhibitor tocilizumab and corticosteroid. In our case, the increased level of interleukin-6 was found in peripheral blood of the patient after radiotherapy following anti-PD-1 drug treatment. The case in our report developed coagulation disorders (thrombocytopenia), nervous system injury (tremor), and acute renal failure, and recombinant human anti-IL-6 receptor monoclonal antibodies tocilizumab, gamma globulin, and methylprednisolone were used to treat, which were successful.
To be noted, the patient in our report developed CRS during radiotherapy following the PD-1 inhibitor application, indicating that the PD-1 inhibitor may still cause severe CRS, especially during the radiotherapy period. And the potential risk of severe CRS may be the result of radiotherapy, which may induce cytokine release. The abscission effect is an immune-mediated tumor regression phenomenon (24,25) away from radiation lesions. Immune checkpoint inhibitors, combined with radiotherapy, have been observed in patients with melanoma (26). Radiation may contribute to and enhance the systemic immune response of the patient. Radiation-induced immune activation is associated with the induction of tumor-associated antigens in the context of endogenous adjuvants, which helps activate anti-tumor cytotoxic T lymphocytes, induce altered microenvironment, and promote immune response (27,28).
Cytokines are essential roles in immune cell communication, through which immune cells attack targets simultaneously. Always, this causes toxicity to healthy tissues. Nephrotoxicity is rarely reported, with an incidence rate of about 1% (11,12). It usually occurs in weeks to months after anti-PD-1 drug treatment. Most of them are acute tubulointerstitial nephritis (ATIN) (29). Increased specific T cell activation and autoantibody production are both possible pathogeneses of immune-related ATIN (30). Here, the serum creatinine of the patient increased abruptly and diagnosed as stage-3 acute renal injury. These results indicated that for elderly patients with reduced renal function, the acute damage to renal from PD-L1 antibodies should be taken into consideration.
irAEs can also cause inhibition of the blood system (14), for example, thrombocytopenia here. The frequency of hematological toxicity was about 0.5% (10). The frequency of immune thrombocytopenia (IT) among hematological toxicities is about 29% (10). This case presents thrombocytopenia and bone marrow suppression, exogenous supplementation of platelets is ineffective. The other side effect of the anti-PD-1 drug, here, was diarrhea, commonly observed (8–19%) (31,32). Immune associated pneumonia caused by anti-PD-1 drugs mainly manifests as nonspecific interstitial pneumonia (33,34). Chest CT examination often shows organ pneumonia and multiple lesions in both lungs, including ground glass change, a grid-like change, pulmonary consolidation, etc. (35). Here, the patient presented intermittent cough and expectoration, and the imaging results showed inflammatory lesions of the lung. However, we cannot exclude the possibility that nosocomial infection causes it or the application of glucocorticoids.
In managing and treating CRS patients, Daniel W. Lee recommended that, in addition to methylprednisolone therapy, a cytokine antagonist (anti-IL-6 receptor monoclonal antibody) should be used as the first-line treatment, considering IL-6 is a central mediator of toxicity in CRS (16). The management of anti-PD-1 associated irAEs is usually on clinical experience and so far, depends on glucocorticoids. The American Society of Clinical Oncology clinical practice guidelines recommend that for some refractory cases, in addition to the application of high-dose glucocorticoids (glucocorticoids should be gradually reduced after at least 4–6 weeks), other immunomodulators are also needed. These immunomodulators include infliximab, tumor necrosis factor inhibitor, mycophenolate mofetil, anti-thymocyte globulin, a calcineurin inhibitor, methotrexate, immunoglobulin for injection or plasma exchange, etc. (36,37). Here, a combination of glucocorticoid, immunomodulator, and cytokine antagonist was used. The strategy has been confirmed to improve the condition. In the current, it was noted that even though the patient developed a typical CRS after 20 Gy/10 times radiotherapy, the chest CT examination immediately found that the esophageal lesions were significantly reduced, almost reaching complete response. The previous three chemotherapies, combined with six immunotherapies, did not achieve a noticeable effect, indicating that radiotherapy combined with immune therapy, may have a superimposed effect. This view is consistent with previous reports (24-28). Therefore, radiotherapy combined with immunotherapy can be used as a novel and effective treatment modality for some patients.
There are some limitations to this study. The PD-1 expression was not detected before the treatment due to the limited sample. Also, the long-term follow-up was on agenda but no data has been collected.
Conclusions
We report an ESC case of CRS and irAEs with multiple organ injury induced by radiotherapy following anti-PD-1 drugs. For anti-PD-1 treatment, it is necessary to evaluate and find immune-related risk factors as early as possible. After the occurrence of CRS and irAEs, comprehensive examination, symptomatic support treatment, and continuous monitoring can help minimize the damage to related side effects.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1310
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1310). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng B, Wang J, Cai W, et al. Changes in the tumor immune microenvironment in resected recurrent soft tissue sarcomas. Ann Transl Med 2019;7:387. [Crossref] [PubMed]
- Isaacsson Velho P, Antonarakis ES. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol 2018;11:475-86. [Crossref] [PubMed]
- Lipson EJ, Forde PM, Hammers HJ, et al. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin Oncol 2015;42:587-600. [Crossref] [PubMed]
- Xu XJ, Tang YM. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett 2014;343:172-8. [Crossref] [PubMed]
- Rotz SJ, Leino D, Szabo S, et al. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer 2017. [Crossref] [PubMed]
- Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013;121:5154-7. [Crossref] [PubMed]
- Hassel JC. Ipilimumab plus nivolumab for advanced melanoma. Lancet Oncol 2016;17:1471-2. [Crossref] [PubMed]
- Weber JS, Yang JC, Atkins MB, et al. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol 2015;33:2092-9. [Crossref] [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer 2019;122:72-90. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Murakami N, Borges TJ, Yamashita M, et al. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin Kidney J 2016;9:411-7. [Crossref] [PubMed]
- Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017;5:8. [Crossref] [PubMed]
- Dimitriou F, Matter AV, Mangana J, et al. Cytokine Release Syndrome During Sequential Treatment With Immune Checkpoint Inhibitors and Kinase Inhibitors for Metastatic Melanoma. J Immunother 2019;42:29-32. [Crossref] [PubMed]
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-95. [Crossref] [PubMed]
- Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017;5:8. [Crossref] [PubMed]
- Tabei A, Watanabe M, Ikeuchi H, et al. The Analysis of Renal Infiltrating Cells in Acute Tubulointerstitial Nephritis Induced by Anti-PD-1 Antibodies: A Case Report and Review of the Literature. Intern Med 2018;57:3135-9. [Crossref] [PubMed]
- Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol 2016;17:188. [Crossref] [PubMed]
- Shirali AC, Perazella MA, Gettinger S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am J Kidney Dis 2016;68:287-91. [Crossref] [PubMed]
- Escandon J, Peacock S, Trabolsi A, et al. Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor. J Immunother Cancer 2017;5:3. [Crossref] [PubMed]
- Uchida A, Watanabe M, Nawata A, et al. Tubulointerstitial nephritis as adverse effect of programmed cell death 1 inhibitor, nivolumab, showed distinct histological findings. CEN Case Rep 2017;6:169-74. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Golden EB, Pellicciotta I, Demaria S, et al. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2012;2:88. [Crossref] [PubMed]
- Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia 2010;15:411-21. [Crossref] [PubMed]
- Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638-47. [Crossref] [PubMed]
- Qiu W, Zheng K, Wang H, et al. Clinical Diagnosis and Treatment Recommendation of Immune-related Adverse Renal Events Related to Immune Checkpoint Inhibitor. Zhongguo Fei Ai ZaZhi 2019;22:645-8. [PubMed]
- Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119:1675-82. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Nishino M, Sholl LM, Hodi FS, et al. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. N Engl J Med 2015;373:288-90. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res 2016;4:289-93. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Thompson JA. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract 2018;14:247-9. [Crossref] [PubMed]
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


