Outcome of twin pregnancy in a patient with Gitelman syndrome: a case report and literature review
Introduction
Gitelman syndrome (GS), first described in 1966, is a rare autosomal-recessive disease, with the clinical syndrome of hypokalemia, hypomagnesemia, hypocalciuria, and metabolic alkalosis. It is caused by inactivating mutations in the SLC12A3 gene on chromosome 16 (16q13) for the thiazide-sensitive sodium-chloride cotransporter in the distal renal tubule. The incidence of GS is about ~25 people per million (1). Usually, patients are asymptomatic, but some patients may have muscle weakness, tetany, salt craving, thirst, nocturia, paresthesia, and abdominal pain (2). Little information is available about the impact of GS to maternal physiologic adaptation and fetal outcome. Here, we describe the management and pregnancy outcome of monochorionic twin pregnancy in a patient with GS. We present the following case in accordance with the CARE Reporting Checklist (available at http://dx.doi.org/10.21037/apm-19-299).
Case presentation
A 25-year-old woman, conceived monochorionic twins by in vitro fertilization embryo transfer (IVF-ET), gravid 1, para 0, visited our perinatal center at 12+2 weeks of gestation. At her booking visit at 11+3 weeks, physical examination was normal. Results of thyroid function were thyroid-stimulating hormone (TSH) 0.008 mIU/L, free tetraiodothyronine (FT4) 22.05 pmol/L, free triiodothyronine (FT3) 8.66 pmol/L, human thyroglobulin (HTG) 31.59 µg/L, thyroglobulin antibody (TgAb) 12.34 IU/ml, thyroid peroxidase antibody (TPOAb) <5.0 IU/mL, thyrotropin receptor antibody (TRAb) 1.15 IU/mL. Electrolytes were Na+: 131 mmol⁄ L, K+: 2.5 mmol/L, HCO3-: 31 mmol/L, Mg2+: 1.0 mmol/L and Cl−: 92 mmol/L. The patient was asymptomatic, no muscle weakness, tetany, abdominal pain and vomiting. Diuretic or anti-emetic use was denied. Fetal ultrasound revealed normal monochorionic diamniotic twin. There was no history of renal disease in the family. The patient was admitted into internal medicine department due to abnormal thyroid function and hypokalemia. Electrocardiogram (ECG) revealed normal.
Ultrasound in abdomen, thyroid and urological were all normal. Twenty-four -hour urine electrolytes showed Cl− 274.6 mmol/24 h (range, 140–250 mmol/24 h), K+ 180.48 mmol/24 h (range, 40–80 mmol/24 h), Ca2+ 0.22 mmol/24 h (range, 2.5–7.5 mmol/24 h), and Mg2+ 4.25 mmol/24 h (range, 3.0–5.0 mmol/24 h). Blood exam results showed plasma renin activity (PRA) >12.0 ng/mL·h, AT-II534.38 ng/mL·h, aldosterone (ALD) 65.61 ng/dL. A diagnosis of GS was made. Daily oral potassium chloride solution and potassium magnesium aspartate were supplemented. The patient was asymptomatic and discharged 18 days on oral K+ and Mg2+. Despite oral KCl and potassium magnesium aspartate, K+ failed to normalize, range 2.5–2.9 mmol/L.
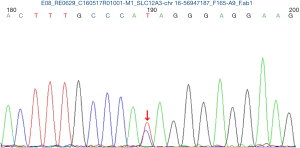
Close monitoring was then performed to the mother and the fetuses. Oral potassium chloride solution and potassium magnesium aspartate were still supplemented. Although maternal blood K+ failed to normalize, range 2.6–3.33 mmol/L in the following pregnancy, she did not develop peripheral paraesthesia, decreased sensation, muscle weakness and arrhythmia etc. Both fetal biometric parameters of the ongoing pregnancy were normal. The couple refused to exam the chromosome of the twins by amniocentesis. Maternal peripheral blood sample was achieved to analysis the SLC12A3 gene and the result revealed that the patient had heterozygous mutations at 988 ATA codon in exon 26, which convert isoleucine to threonine (I988T) (Figure 1). The diagnosis of GS was confirmed. ECG during pregnancy was with no cardiac dysrhythmias or changes in the QT interval on electrocardiographic tracing.
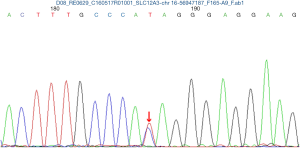
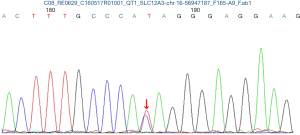
The pregnancy was uneventful and she remained asymptomatic despite persistent hypokalemia. At 36+4 weeks of gestation, two babies were delivered by elective cesarean section under combined spinal epidural anesthesia, first female baby weighing 2,380 g with Apgar scores of 10 and 10 at 1 and 5 min, second female baby weighing 2,210 g with Apgar scores of 10 and 10 at 1 and 5 min. Two neonates were transferred to the NICU due to maternal hypokalemia. Both cord blood sample was achieved to examine the SLC12A3 gene analysis and the results in two babies also revealed heterozygous mutations at 988 ATA codon in exon 26, which convert isoleucine to threonine (I988T) (Figures 2 and 3). GS of both babies was diagnosed. Electrolytes profile of both neonates were normal and discharged 3 days later. The postoperative period was uneventful and the patient were discharged 3 days after the operation on oral K+ and symptom free. Until now, the mother and the twins are all in good health condition during three years follow-up. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
GS is a congenital renal tubular defect resulting from loss-of-function mutations in the thiazide-sensitive Na-Cl cotransporter (SLC12A3) gene (3), which affects the apical membrane of the distal convoluted tubule of the renal system. The exact mechanism remains unclear.
GS incidence is about ~25 per million. It is one of the most frequent inherited renal tubular disorders (1). Due to its different phenotypes and asymptomatic, GS is often diagnosed in adolescence or early adulthood. The clinical manifestation include muscle cramps, tiredness, tetany, nausea, vomiting, polyuria, nocturnal diuresis, heart abnormalities etc. (4,5). In most cases, GS results from inactivating mutations in SLC12A3 gene, which encodes the NCC and consists of 26 exons and is located on the long arm of chromosome 16 (16q13) (6). To date, more than 100 different mutations have been identified in SLC12A3 gene in GS. Upon the literatures, most of point mutations lead to complete loss of expression and cotransporter activity, whereas some of the missense mutations in SLC12A3 exhibit partial function (7-10). Therefore, patients with GS present with multiple phenotypes.
In our case, SLC12A3 gene analysis in mother and two babies revealed heterozygous mutations at 988 ATA codon in exon 26, which convert isoleucine to threonine (I988T), this heterozygous mutation was also reported by Jang et al. (10).
Bartter syndrome is also an inherited hypokalemic salt-losing renal tubular disorder. It also has the presentation of hypokalemia, metabolic alkalosis and hyperreninemic, hyperaldosteronism, which results the difficulty in management of GS and Bartter syndrome. However, Bartter syndrome is related with mutations in some transporters in the thick ascending limb of Henle’s loop and presents with normal serum magnesium and high urinary calcium, which is different from hypomagnesemia and hypocalcinuria in GS (3,11). In our case, the patient presented with hypokalemia, hypomagnesemia and hypocalcinuria, GS was diagnosed by clinical outcomes, then confirmed by the mutations in the SLC12A3 gene.
During pregnancy, renal hemodynamics change with increased glomerular filtration rate (GFR) and renal blood flow, which is associated with increased tubular reabsorption to maintain physiologic electrolyte balance. Urinary potassium and magnesium loss are physiologic processes during pregnancy due to increased aldosterone levels. Healthy pregnant women can tolerate this increased urinary loss. As a result of tubular defect, GS can result in uncontrolled potassium and magnesium loss. During pregnancy, emesis gravidarum and fetal demands for potassium can further aggravate hypokalemia. The goal of therapy in pregnant with GS is to get rather serum levels of potassium and magnesium that allow patients to be asymptomatic and for a successful perinatal outcome (12).
There are only a few cases describing the impact of GS on pregnancy and the fetus. We performed an extensive search of the literature about GS in pregnancy and full-length articles or letters in peer-reviewed scientific literature in English and Chinese were reviewed. To the best of our knowledge, 20 articles (4,5,12-29) had been published before 2014, 8 articles (30-37) published after 2014. Case reports on GS in pregnancy and most of them show favorable outcomes.
There were 57 reported pregnancies in 41 women affected with GS. Fifteen women with GS were confirmed through genetic testing. Among the 57 pregnancies, there were 3 spontaneous abortions, 4 fetal demise and one still birth. Among the remaining 49 pregnancies, 13 pregnancies were with preterm delivery, 36 pregnancies with term delivery, which resulted in 49 live births. One pregnant developed ventricular fibrillation on day 9 of hospitalization and was complicated by a refractory cardiac arrest despite advanced cardiac life support (36). Most of the case reports show no adverse fetal outcomes. In our case, it was the successful outcome of monochorionic diamniotic (MCDA) twin pregnancy in a patient with GS.
Most authors agree that monitoring of the electrolytes involved is necessary and proper supplementation of both potassium and magnesium is indicated. Upon the literatures, most patients with GS need magnesium and potassium supplementation in pregnancy. Close laboratory monitoring and aggressive electrolyte repletion can contribute to a successful pregnancy outcome (21). Choice of IV electrolyte replacement versus oral or some combination should be individualized based on low electrolyte levels and/or symptoms exacerbation. Talaulikar’s and Kwan’s reports suggested intravenous supplementation may not be necessary to achieve a good fetal outcome (12,23). However, transition to IV electrolyte repletion should be considered when oral doses are not sustaining goal electrolyte levels or the side effect profile limits increased dosages.
The use of the aldosterone antagonists, spironolactone and eplerenone, or the epithelial sodium channel inhibitor, amiloride, has been proposed. Spironolactone is pregnancy class D although there are reports in the literature of use during pregnancy without adverse effects. Eplerenone and amiloride are pregnancy class B, each with successful use reported in patients with GS (4,18,22). In our patient, persistently low levels of serum potassium and magnesium were noted despite the intensive daily oral supplementation. Nevertheless, the pregnancy outcome was excellent. Due to the potential fetal risks, we chose not to use this therapy in our patient.
Often patients with this syndrome do not present until late childhood or early adulthood. They are actually often asymptomatic This case is the first case of pregnant with MCDA twin pregnancy in GS. The mother and twin babies are followed and in good health. The strength of this report is to study the perinatal outcome of twin pregnancy complicated with GS and SLC12A3 gene analysis was performed in mother and two babies. It is regretting that SLC12A3 gene analysis cannot be performed in other members of the family due to their refusal.
GS is a rare medical condition affecting pregnancy. Overall, GS does not have adverse effect on the pregnancy outcome in most cases provided regular supplementation is used to relieve maternal symptoms. Genetic counselling is of value but antenatal fetal diagnosis of GS is not advised based on the good prognosis in the majority of patients (1). Close fetal serial surveillance should monitor fetal growth and liquor. Management of these challenging patients by multidisciplinary team, including obstetricians, endocrinologists, anesthetists, neonatologists and geneticists, is critical to optimizing outcomes for mother and fetus.
Conclusions
In summary, based on the published reports and our case, it is concluded that pregnant women with GS may have a favorable pregnancy and neonatal outcome if they are with close follow-up, frequent electrolyte evaluation and adequately replenishment with potassium and magnesium. Management by multidisciplinary team is critical to optimizing outcomes for mother and fetus.
Acknowledgments
We feel grateful to the doctors and staff who have been involved in this work.
Funding: This Study was supported by the Academic and Technical Leader’s Foundation of Sichuan Province (No. 2017-919-25), the Science Foundation of Sichuan Province (No. 2018FZ0041) and National Natural Science Foundation of China (No. 81571446).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-299). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent to publication was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Knoers NV, Levtchenko EN. Gitelman syndrome. Orphanet J Rare Dis 2008;3:22. [Crossref] [PubMed]
- Nakhoul F, Nakhoul N, Dorman E, et al. Gitelman's syndrome: a pathophysiological and clinical update. Endocrine 2012;41:53-7. [Crossref] [PubMed]
- Riveira-Munoz E, Chang Q, Godefroid N, et al. Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J Am Soc Nephrol 2007;18:1271-83. [Crossref] [PubMed]
- Mathen S, Venning M, Gillham J. Outpatient management of Gitelman's syndrome in pregnancy. BMJ Case Rep 2013;2013:bcr2012007927.
- Daskalakis G, Marinopoulos S, Mousiolis A, et al. Gitelman syndrome-associated severe hypokalemia and hypomagnesemia: case report and review of the literature. J Matern Fetal Neonatal Med 2010;23:1301-4. [Crossref] [PubMed]
- Glaudemans B, Yntema HG, San-Cristobal P, et al. Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome. Eur J Hum Genet 2012;20:263-70. [Crossref] [PubMed]
- Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 2005;85:423-93. [Crossref] [PubMed]
- De Jong JC, Van Der Vliet WA, Van Den Heuvel LP, et al. Functional expression of mutations in the human NaCl cotransporter: evidence for impaired routing mechanisms in Gitelman's syndrome. J Am Soc Nephrol 2002;13:1442-8. [Crossref] [PubMed]
- Wang X, Ding Y, Liu Q, Yang G.A novel compound heterozygous mutation of SLC12A3 gene in a Chinese pedigree with Gitelmansyndrome. Endocrine. 2019VN. Available online: https://doi.org/ [Crossref]
- Jang HR, Lee JW, Oh YK, et al. From bench to bedside: diagnosis of Gitelman's syndrome -- defect of sodium-chloride cotransporter in renal tissue. Kidney Int 2006;70:813-7. [Crossref] [PubMed]
- Naesens M, Steels P, Verberckmoes R, et al. Bartter's and Gitelman's syndromes: from gene to clinic. Nephron Physiol 2004;96:65-78. [Crossref] [PubMed]
- Talaulikar GS, Falk MC. Outcome of pregnancy in a patient with Gitelman syndrome: a case report. Nephron Physiol 2005;101:35-8. [Crossref] [PubMed]
- Jones JM, Dorrell S. Outcome of Two Pregnancies in a Patient with Gitelman's Syndrome-A Case Report. J Matern Fetal Investig 1998;8:147-8. [PubMed]
- De Bustros A, Aleppo G, Zikos D. Hypokalemia in pregnancy: Clue to gitelman syndrome. Endocrinologist 2001;11:447-50. [Crossref]
- Basu A, Dillon RD, Taylor R, et al. Is normalisation of serum potassium and magnesium always necessary in Gitelman Syndrome for a successful obstetric outcome? BJOG 2004;111:630-4. [Crossref] [PubMed]
- Srinivas SK, Sukhan S, Elovitz MA. Nausea, emesis, and muscle weakness in a pregnant adolescent. Obstet Gynecol 2006;107:481-4. [Crossref] [PubMed]
- de Haan J, Geers T, Berghout A. Gitelman syndrome in pregnancy. Int J Gynaecol Obstet 2008;103:69-71. [Crossref] [PubMed]
- de Arriba G, Sanchez-Heras M, Basterrechea MA. Gitelman syndrome during pregnancy: a therapeutic challenge. Arch Gynecol Obstet 2009;280:807-9. [Crossref] [PubMed]
- Shanbhag S, Neil J, Howell C. Anaesthesia for caesarean section in a patient with Gitelman's syndrome. Int J Obstet Anesth 2010;19:451-3. [Crossref] [PubMed]
- McCarthy FP, Magee CN, Plant WD, et al. Gitelman's syndrome in pregnancy: case report and review of the literature. Nephrol Dial Transplant 2010;25:1338-40. [Crossref] [PubMed]
- Lakhi N, Jones J, Govind A. Fetal demise despite normalisation of serum potassium in Gitelman syndrome. Case report and literature review. Aust N Z J Obstet Gynaecol 2010;50:301-2. [Crossref] [PubMed]
- Morton A, Panitz B, Bush A. Eplerenone for gitelman syndrome in pregnancy. Nephrology (Carlton) 2011;16:349. [Crossref] [PubMed]
- Kwan TK, Falk MC. Second pregnancy outcome in a patient with Gitelman syndrome without the use of parenteral electrolyte supplementation. Aust N Z J Obstet Gynaecol 2011;51:94-5. [Crossref] [PubMed]
- Mascetti L, Bettinelli A, Simonetti GD, et al. Pregnancy in inherited hypokalemic salt-losing renal tubular disorder. Obstet Gynecol 2011;117:512-6. [Crossref] [PubMed]
- Raffi F, Fairlie FM, Madhuvrata P, et al. Pregnancy with Gitelman's syndrome. Obstet Med 2011;4:39-41. [Crossref] [PubMed]
- Zhu Y, Zhang X. Pregnancy with Gitelman syndrome:one case. Chin J Perinat Med 2011;14:768-9.
- Moustakakis MN, Bockorny M. Gitelman syndrome and pregnancy. Clin Kidney J 2012;5:552-5. [Crossref] [PubMed]
- Calo LA, Caielli P. Gitelman's syndrome and pregnancy: new potential pathophysiological influencing factors, therapeutic approach and materno-fetal outcome. J Matern Fetal Neonatal Med 2012;25:1511-3. [Crossref] [PubMed]
- Shinar S, Gal-Oz A, Weinstein T, et al. Gitelman syndrome during pregnancy – from diagnosis to treatment: a case series and review of the literature. Case Reports in Perinatal Medicine 2013;3:39-43.
- Waguespack DR, Kasekar R, Abdel-Kader K, et al. Two cases of successful pregnancy in patients with Gitelman's syndrome. Clin Nephrol 2015;84:301-6. [Crossref] [PubMed]
- Koudsi L, Nikolova S, Mishra V. Management of a severe case of Gitelman syndrome with poor response to standard treatment. BMJ Case Rep 2016;2016.
- Chen G. Qiao c. Second pregnancy outcome in a patient with Gitelman syndrome: a case report and literature review. Prog Obstet Gynecol 2016;25:719-20.
- Song DL. Gitelman syndrome unexpectedly diagnosed at early pregnancy and the gene mutation analysis of the family: one case report. Chinese Journal of Endocrinology and Metabolism 2016;32:869-71.
- Nand N, Deshmukh AR, Mathur R, et al. Gitelman Syndrome: Presenting During Pregnancy with Adverse Foetal Outcome. J Assoc Physicians India 2016;64:104-5. [PubMed]
- Lee M, Kim DI, Lee KH, et al. HELLP syndrome in a pregnant patient with Gitelman syndrome. Kidney Res Clin Pract 2017;36:95-9. [Crossref] [PubMed]
- Elkoundi A, Kartite N, Bensghir M, et al. Gitelman syndrome: a rare life-threatening case of hypokalemic paralysis mimicking Guillain-Barre syndrome during pregnancy and review of the literature. Clin Case Rep 2017;5:1597-603. [Crossref] [PubMed]
- Tu J, Liu Q, Yin C. Pregnancy with Gitelman syndrome: two cases. J Pract Obstet Gynecol 2018;34:399-400.