The efficacy and safety of azithromycin in chronic respiratory diseases related cough
IntroductionOther Section
Cough is one of the most common respiratory symptom, which affects 8–10% of the adult population, leading to seek medical care in western countries as well as in China (1-3). Chronic respiratory diseases causing cough include asthma, eosinophilic bronchitis, postnasal drip syndrome or rhinosinusitis, chronic obstructive pulmonary disease (COPD), bronchiectasis, etc. (1,4,5). The management of these patients should be aimed at pathogeny cure. Several treatments for chronic respiratory diseases with cough have been identified over the past decades, including inhaled corticosteroids (ICS), neuromodulatory therapies, non-pharmacologic therapies and other therapies (6-8).
Generally, ICS, β2 adrenergic receptor agonist and muscarinic receptor antagonist have been proposed to be the basic treatments for chronic respiratory diseases, including asthma and COPD (9,10). After inhalers, advanced therapies are also available, including phosphodiesterase inhibitors, leukotriene receptor antagonist, N-acetylcysteine, even immunotherapy (monoclonal antibodies) (9,10). However, their treatment response often limited in case of refractory cough (8,11,12). Therefore, better approaches to chronic respiratory diseases with cough are needed.
Besides antibacterial effects, azithromycin, as a kind of macrolide antibiotics, has been reported to have anti-inflammatory and immunomodulatory effects in chronic airway inflammatory diseases, including bronchiectasis, COPD, asthma (13-16). A recently meta-analysis assessed the efficacy and safety of long-term add-on treatment of azithromycin in asthma (17). They mainly focused on the therapeutic effect of azithromycin in lung function [forced expiratory volume in one second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF)], symptom control, quality of life [Asthma Control Questionnaire (ACQ), Asthma Quality of Life Questionnaire (AQLQ)] and airway inflammation (16,17). However, rare attention was paid to the cough symptom of such airway diseases, which had greater impact on quality of life for some patients than other symptoms (18,19). Therefore, we did a systematic review aiming to provide a summary of the efficacy and safety of azithromycin in patients of chronic respiratory diseases with cough. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-119).
MethodsOther Section
Inclusion and exclusion criteria
We included prospective randomized controlled trials (RCTs) involving patients of chronic respiratory diseases with cough. Azithromycin should be administrated as compared with placebo or in combination with other treatments as compared with other treatments alone. We limited publications to the English language. We excluded crossover trials, abstract publications, before-after studies, conference presentations, editorials and case reports. No statement on medical ethics is required for the systematic review and meta-analysis.
Search strategy
To increase the sensitivity of the search strategy, we combined the terms “azithromycin” with “cough” as key words or Medical Subject Headings (MeSH) terms. Four databases, including PubMed, EMBASE, Cochrane, and Web of Science, were searched from electronic databases inception to October, 1st, 2019. We systematically screened abstracts and full text articles for studies that met our eligibility criteria. The process was performed by two researchers (J Zhou and F Yi) independently.
Outcome assessment
The primary outcome of this review was the improvement of cough, assessed by Leicester Cough Questionnaire (LCQ), the Cough Quality-of-Life Questionnaire (CQLQ) and the cough visual analogue scale (VAS). The secondary outcome was the incidence of adverse effects by azithromycin for the treatment of cough.
Data abstraction
Two investigators (J Zhou and F Yi) reviewed and abstracted data from each retrieved article and supplement independently. Discrepancies were resolved by discussion and consensus.
Quality assessment
The quality of all included trials was reviewing by the details in their method sections and their supplemental materials. The trial quality was appraised by using the Cochrane collaboration tool for assessing risk of bias (RoB) (20), including assessment of random sequence generation, allocation concealment, blinding (of interventions, outcome measurement or assessment), selective reporting bias and incomplete outcome data. For each criterion, we appraised the RoB to be either of low, high, or unclear risk. Two researchers (J Zhou and F Yi) assessed the trial quality independently and disagreements were resolved by discussion.
Assessment of heterogeneity
We used the I2 statistic to evaluate the heterogeneity on pooled data. If an I2 value was greater than 50%, then a substantial heterogeneity was indicated (20). Fixed-effects model was used to pool data when heterogeneity was insignificant. When significant heterogeneity was found, then the random effects models would be used to pool data.
Statistical analysis
The changes in LCQ score and adverse events rates were analysed in this meta-analysis. Continuous data and categorical data were pooled by using the mean difference (MD) and risk ratio (RR), with the 95% confidence intervals (CIs). The comparison of the outcome between the azithromycin and placebo was conducted with Review Manager (RevMan) Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014), and two-sided P values less than 0.05 were considered to be statistically significant.
ResultsOther Section
Characteristics of included trials
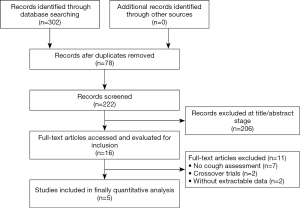
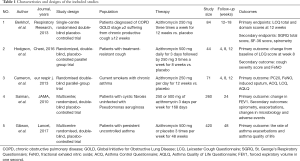
We identified 302 potentially eligible studies. After exclusion of duplicate and irrelevant articles, 16 studies were retrieved to be reviewed in greater detail. Of these, we excluded 11 studies that did not meet our eligibility criteria and thus included 5 trials in our review (Figure 1). All of the included studies were designed as randomised, double-blind, placebo-controlled clinical trials. Of the 5 RCTs, two studies were conducted in patients with asthma (15,21), one study was conducted in patients with COPD (22). Hodgson et al. conducted a study in patients with bronchial hyperresponsiveness (23). Saiman et al. conducted a study in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa (24). The characteristics of the studies are shown in Table 1.
Full table
RoB of included studies
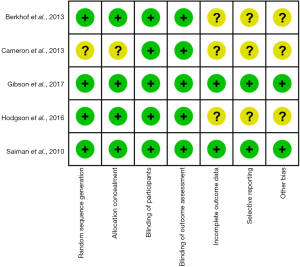
All included trials were reported to be low risk of performance bias and four studies were assessed to be at low RoB with respect to selection bias except for one study for which selection bias was deemed unclear (21). Two trials assessed to be at low RoB with regard to completeness of outcomes data, selective outcomes reporting, and other potential sources of bias (15,24). But the other three trials were considered to be at unclear risk with respect to the above bias (21-23) (Figure 2).
Azithromycin in cough
Cough can be assessed by many ways and the LCQ has been well validated with internal consistency, repeatability and responsiveness (25,26). However, only three of the included trials have reported the change of LCQ (21-23). Compared to placebo, azithromycin intervention had no effect in reducing cough (n=198, MD 0.73; 95% CI: −0.78 to 2.24; P=0.34; I2=71%). Further analysis showed that the trial from Cameron et al. (21) was main source of heterogeneity. When excluding the above trial, the left two trials (22,23) had shown clinically important improvement in LCQ score by azithromycin administration (n=121, MD 1.30; 95% CI: 1.15–1.46; P<0.00001; I2=0%) (Figure 3).

There other two studies did not assessed cough by LCQ. The study from Saiman et al. showed that azithromycin intervention significantly reduced the frequency of cough (−23% treatment difference; 95% CI: −33 to −11; P<0.001) and productive cough (−11% treatment difference; 95% CI: −19 to −3; P=0.01) than those in placebo group (24). Another study from Gibson et al. measured cough by using cough VAS. They found that there was a significant reduction in cough and sputum production VAS in patients using azithromycin (15). No included study measured cough by using CQLQ.
Adverse events
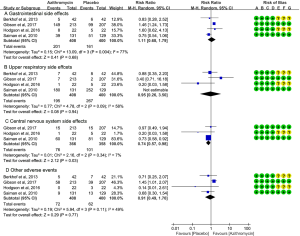
There were four trials report the adverse events during azithromycin intervention (15,22-24). A pooled analysis applied in a random effect model revealed that there was no significant difference in upper respiratory (n=808, RR 1.11; 95% CI: 0.68–1.79; P=0.68), gastrointestinal (n=808, RR 0.95; 95% CI: 0.26–3.50; P=0.94) and other adverse events (n=808, RR 0.91; 95% CI: 0.49–1.70; P=0.77) between the two groups. However, pooled data showed that azithromycin intervention had less central nervous system than the placebo in a fixed effect model (heterogeneity I2=7%, P=0.03) (Figure 4).
DiscussionOther Section
Our systematic review pooled the data of 879 patients of chronic respiratory diseases with cough from five randomised, placebo controlled clinical trials to evaluate the efficacy of azithromycin on cough. It showed that the addition of oral azithromycin to standard care for the associated respiratory diseases resulted in no statistically significant benefit for reducing cough in three eligible trials but with significant heterogeneity. After excluding the substantial heterogeneity trial, we found that addition of oral azithromycin could improve LCQ score and cough VAS score, reduce the incidence of cough. In addition, the treatment was well-tolerated. No significant side effect of azithromycin treatment was found. And pooled data showed that azithromycin intervention had less central nervous system than the placebo.
Azithromycin, a macrolide antibiotic with a broader microbial spectrum and fewer side effects was recommended in many indications. However, azithromycin was reported to play an important role in anti-inflammatory and immunomodulatory activities independent of their antimicrobial activity in chronic respiratory diseases, including bronchiectasis, COPD, asthma etc. (13-15). More importantly, azithromycin was demonstrated to prevent exacerbations in chronic respiratory diseases (27). A significant decrease in the exacerbation rate of cystic fibrosis patients with chronic Pseudomonas infection was reported after using azithromycin (28,29). Maintenance treatment with azithromycin significantly decreased the frequency of exacerbations and improved quality of life of COPD patients (14,30). Azithromycin also succeeded in reducing the exacerbation rate of persistent uncontrolled asthma (15). Azithromycin was considered to be the holy grail to prevent exacerbations in chronic respiratory diseases (27).
Cough, as one of the most common symptoms in chronic respiratory diseases, was usual to be overlooked. Cough could be difficult to treat and contributed to poor quality of life, with a significant impact on physical, psychological, and social activities (19,23). Based on a clinical founding of a dramatic improvement in patients with chronic respiratory diseases treated with azithromycin, we mainly focused on the efficacy and safety of azithromycin in symptoms of cough. Therefore, we conducted a systematic review of the literatures and the pooled data had shown that azithromycin had no statistically significant benefit for reducing cough but with significant heterogeneity. The trial from Cameron et al. (21) might be the main source of heterogeneity. There were unclear risks of selection bias (including assessment of random sequence generation, allocation concealment), selective reporting bias and attrition bias due to lack of such information in the manuscript. However, the other two trials (22,23) had shown low risk of selection bias, performance bias and detection bias. And the pooled data of the two trials showed a clinically important improvement in LCQ score by azithromycin administration. Similarly, another two eligible trials with low risks of bias also showed that azithromycin intervention significant reduced both the incidence of cough (24) and the cough VAS score (15). Therefore, additional azithromycin administration maybe benefits for patients of chronic respiratory diseases with cough in a well-designed and high-quality trial.
Eosinophilic inflammation is an important cause of cough symptom (31,32). The possible mechanism of azithromycin in relieving cough may be an anti-inflammatory effect both systemically and locally within the airway. Azithromycin has complex immunomodulatory effects on eosinophilic airway inflammation and inhibition of cytokine production (33,34). Moreover, azithromycin could suppress CD4+ T-cell activation by direct modulation of mTOR activity, which inhibited eosinophil differentiation and allergic inflammation (35,36). Therefore, additional azithromycin administration maybe benefits for patients of chronic respiratory diseases with cough.
For the safety analysis, we classified nasal congestion, pharyngolaryngeal pain, rhinorrhea, common cold, cough into upper respiratory side effects. Vomiting, upper abdominal pain, nausea, diarrhea, ulcus ventriculi, abnormal liver function tests were classified into gastrointestinal side effects. Pyrexia, fatigue, vertigo, tinnitus, hearing loss headache were classified into central nervous system side effects. Other adverse events included rash, allergy, oral thrush, QTc prolongation, back pain, rib pain, myocardial infarction, supraventricular tachycardia, heart failure, hyperhidrosis, malaise etc. We found that there was no significant difference in upper respiratory, gastrointestinal and other adverse events of azithromycin treatment in patients of chronic respiratory diseases with cough. Moreover, azithromycin intervention had less central nervous system than the placebo. Therefore, additional azithromycin administration may be safety for patients of chronic respiratory diseases with cough.
There were several limitations in this meta-analysis. Firstly, the eligible trial was limited and the sample size was relatively small, the conclusion of our analysis might be carefully made before transferring to a large cough population. Secondly, potential publication bias may not be ignored and we failed to identify potential unpublished negative studies that may alter the outcome. In addition, the heterogeneity derived from the design of an RCT, causes of cough, baseline treatment, the dosage and period of azithromycin treatment among studies, which might contribute to the inconsistency. Finally, for the safety analysis, side effects were classified into certain scale such as upper respiratory and gastrointestinal adverse events rather than one side effect by one comparison, which may cover certain significant side effects.
ConclusionsOther Section
Our systematic review and meta-analysis found that the addition of oral azithromycin to standard care for the associated respiratory diseases resulted in statistically significant benefit for patients with cough. Azithromycin administration was safety and probably showed less central nervous system side effects for patients with cough. However, more RCTs with large sample size should be conducted to establish the precise role of azithromycin in the chronic respiratory diseases related cough treatment.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the PRISMA reporting Checklist. Available at http://dx.doi.org/10.21037/apm-20-119
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-119). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008;371:1364-74. [Crossref] [PubMed]
- Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479-81. [Crossref] [PubMed]
- Lai K, Pan J, Chen R, et al. Epidemiology of cough in relation to China. Cough 2013;9:18. [Crossref] [PubMed]
- Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013;143:613-20. [Crossref] [PubMed]
- Bergamini M, Kantar A, Cutrera R, et al. Analysis of the Literature on Chronic Cough in Children. Open Respir Med J 2017;11:1-9. [Crossref] [PubMed]
- Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ 2015;351:h5590. [Crossref] [PubMed]
- Michaudet C, Malaty J. Chronic Cough: Evaluation and Management. Am Fam Physician 2017;96:575-80. [PubMed]
- Ryan NM, Vertigan AE, Birring SS. An update and systematic review on drug therapies for the treatment of refractory chronic cough. Expert Opin Pharmacother 2018;19:687-711. [Crossref] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2019. Available online: https://ginasthma.org/reports/
- Global strategy for prevention, diagnosis and management of COPD. 2020. Available online: https://goldcopd.org/gold-reports/
- Chamberlain Mitchell SA, Garrod R, Clark L, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax 2017;72:129-36. [Crossref] [PubMed]
- Wei W, Liu R. The efficacy of specific neuromodulators on human refractory chronic cough: a systematic review and meta-analysis. J Thorac Dis 2016;8:2942-51. [Crossref] [PubMed]
- Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:660-7. [Crossref] [PubMed]
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689-98. [Crossref] [PubMed]
- Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:659-68. [Crossref] [PubMed]
- Tian BP, Xuan N, Wang Y, et al. The efficacy and safety of azithromycin in asthma: A systematic review. J Cell Mol Med 2019;23:1638-46. [Crossref] [PubMed]
- Wang X, Luo J, Wang D, et al. The efficacy and safety of long-term add-on treatment of azithromycin in asthma: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e17190. [Crossref] [PubMed]
- Osman LM, McKenzie L, Cairns J, et al. Patient weighting of importance of asthma symptoms. Thorax 2001;56:138-42. [Crossref] [PubMed]
- French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med 1998;158:1657-61. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Cameron EJ, Chaudhuri R, Mair F, et al. Randomised controlled trial of azithromycin in smokers with asthma. Eur Respir J 2013;42:1412-5. [Crossref] [PubMed]
- Berkhof FF, Doornewaard-ten Hertog NE, Uil SM, et al. Azithromycin and cough-specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respir Res 2013;14:125. [Crossref] [PubMed]
- Hodgson D, Anderson J, Reynolds C, et al. The Effects of Azithromycin in Treatment-Resistant Cough: A Randomized, Double-Blind, Placebo-Controlled Trial. Chest 2016;149:1052-60. [Crossref] [PubMed]
- Saiman L, Anstead M, Mayer-Hamblett N, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010;303:1707-15. [Crossref] [PubMed]
- Birring SS, Spinou A. How best to measure cough clinically. Curr Opin Pharmacol 2015;22:37-40. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Welte T. Azithromycin: The Holy Grail to Prevent Exacerbations in Chronic Respiratory Disease? Am J Respir Crit Care Med 2019;200:269-70. [Crossref] [PubMed]
- Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290:1749-56. [Crossref] [PubMed]
- Mayer-Hamblett N, Retsch-Bogart G, Kloster M, et al. Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial. Am J Respir Crit Care Med 2018;198:1177-87. [Crossref] [PubMed]
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014;2:361-8. [Crossref] [PubMed]
- Brightling CE. Eosinophils, bronchitis and asthma: pathogenesis of cough and airflow obstruction. Pulm Pharmacol Ther 2011;24:324-7. [Crossref] [PubMed]
- Lai K, Chen R, Peng W, et al. Non-asthmatic eosinophilic bronchitis and its relationship with asthma. Pulm Pharmacol Ther 2017;47:66-71. [Crossref] [PubMed]
- Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014;143:225-45. [Crossref] [PubMed]
- Hiles SA, McDonald VM, Guilhermino M, et al. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis. Eur Respir J 2019;54:1901381. [Crossref] [PubMed]
- Ratzinger F, Haslacher H, Poeppl W, et al. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep 2014;4:7438. [Crossref] [PubMed]
- Hua W, Liu H, Xia LX, et al. Rapamycin inhibition of eosinophil differentiation attenuates allergic airway inflammation in mice. Respirology 2015;20:1055-65. [Crossref] [PubMed]