
Treatment response to intrathecal chemotherapy with pemetrexed via an Ommaya reservoir in EGFR-mutated leptomeningeal metastases from non-small cell lung cancer: a case report
Introduction
Leptomeningeal metastasis (LM) occurs in up to 5% of cases with advanced non-small cell lung cancer (NSCLC) (1). The incidence of LM is higher in patients with epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)-targetable mutations as a result of the survival benefits gained via precise molecular targeted therapy (2). Despite the continuous development of traditional treatments, such as EGFR-TKIs, systemic chemotherapy, intrathecal chemotherapy and whole-brain radiotherapy (WBRT), the prognosis for patients with LM from NSCLC remains poor, and the collective median survival time is only 3–11 months (3,4). Most antineoplastic drugs cannot reach effective concentrations due to the blood brain barrier (BBB), which is the main limiting factor in curative treatment.
Intrathecal chemotherapy can overcome the BBB to directly deliver drugs into cerebrospinal fluid (CSF); thus, it is useful for efficient LM treatment (5). Compared with lumbar puncture (LP), Ommaya reservoirs are a more convenient and safer approach for implementing intrathecal chemotherapy. Pemetrexed is a multitargeting antifolate agent, and it has demonstrated antitumor activity against nonsquamous NSCLC (6). Here we report the first case of a patient with LM from EGFR mutation-positive NSCLC who achieved both clinical and CSF cytological responses to intrathecal pemetrexed delivered via an Ommaya reservoir. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-19-521).
Case presentation
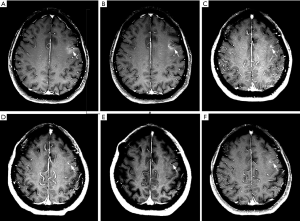
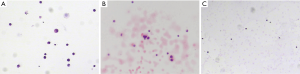
A 57-year-old Asian female nonsmoker was diagnosed with lung adenocarcinoma via positron emission tomography computed tomography (PET-CT) in July 2015. Thoracoscopic resection of lung cancer was implemented, and EGFR testing revealed exon 19 deletion mutations. Gefitinib was applied in August 2015, and the patient obtained stable clinical remission for 28 months. In January 2018, the patient showed symptoms of dizziness, headaches and epileptic seizures. Her plasma carcinoembryonic antigen (CEA) level was clearly elevated. LM was confirmed via magnetic resonance imaging (MRI) (Figure 1) and positive CSF cytology (Figure 2). Osimertinib was administered daily at 80 mg, which led to symptomatic remission and a decrease in the CEA level and of the lesion size (Figure 1). Six months later, she suffered severe headaches, neck stiffness and gradually aggravated lower limb fatigue. An additional CSF test detected a small number of malignant cells (Figure 2) and an EGFR exon 19 deletion and no T790M mutation. Osimertinib (160 mg daily) was started in August 2018. Her CEA level remained stable, and a repeat MRI showed a shrunken lesion (Figure 1); however, there was no significant improvement in the LM symptoms. To further enhance the local control of LM, an intraventricular Ommaya reservoir (Medtronic Inc., Goleta, USA) was installed in November 2018. Pemetrexed and other adjuvant treatments were implemented on days 1 and 8 every 21 days via the Ommaya reservoir. She also continued to take 160 mg of osimertinib daily. The specific procedure was as follows: after adequate partial scalp disinfection, a 24G scalp vein needle (Kindly Medical Instruments Co., Ltd., Shanghai, China) was inserted into the Ommaya reservoir, and 2–5 mL of CSF (1 mL/min) was extracted based on pressure. Next, 5 mg of dexamethasone (0.5 mL/min) and 30 mg of pemetrexed dissolved in 1 mL (0.5 mL/min) were sequentially injected. Finally, depakine and antiemetics were intravenously administered to prevent epilepsy and to alleviate gastrointestinal reactions. This combined treatment ameliorated the neurological defects and led to improved CSF cytology (Figure 2) with no notable side effects. At recent follow-ups in March, June and September 2019, MRI examinations revealed that the LM lesion had remained stable (Figure 1). The whole course of diagnosis and treatment was organized as a timeline (Figure 3).
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
Currently, there is no any consensus or standard guidelines for the treatment of LM associated with advanced NSCLC (3). Targeted therapy combined with local treatment comprise the most common therapeutic approach for patients with EGFR mutations. Radiotherapy can be effective for isolated lesions in the brain, whereas whole-brain and craniospinal radiotherapy are inefficacious for LM diffuse lesions, and these approaches are controversial due to the severe marrow suppression and increased mortality that can result (7).
By being able to cross the BBB, intrathecal chemotherapy has great advantages in clearing small leptomeningeal nodular deposits and individual tumor cells floating in CSF (8). The administration route mainly includes Ommaya reservoirs and LP, and the former has the following benefits (9-11). First, this operation is more convenient and safer and reduces patients’ pain and the incidence of infections due to repeated LP. Second, clinicians can drain CSF via the Ommaya reservoir anytime depending on the CSF pressure to readily alleviate the symptoms of intracranial hypertension. The collected CSF can be used to measure drug concentrations and tumor markers, direct the drug dosage, and assess efficacy and prognosis. Finally, chemotherapeutic drugs are directly injected into the cerebral ventricle via the Ommaya reservoir, after which they are slowly released and uniformly distributed as CSF circulates through the brain and spinal cord and drains (12). The local drug concentration can reach 10 times higher than that attainable via LP (5), which ensures more robust antitumor activity and improved survival (13). Therefore, Ommaya reservoirs are the preferred manner of intrathecal chemotherapy.
Currently, Ommaya reservoirs have been successfully used in the treatment of LM from multiple malignant tumors (14-18). Methotrexate is the most commonly chosen and relatively effective medication; however, it is now rarely used in clinical practice due to its clear toxicity and limited survival benefits (5). Pemetrexed has shown significant antitumor activity against NSCLC without notable adverse effects (6). Some studies have demonstrated that pemetrexed cannot efficiently control CNS metastases due to its poor BBB permeability (18,19). Intrathecally administered pemetrexed has been proven to be safe, and this approach can achieve higher and more stable concentrations in the CNS (20,21). Thus, intrathecal chemotherapy with pemetrexed was administered via an Ommaya reservoir after full consultation with the patient and after obtaining written informed consent. This new approach was well tolerated and showed notably efficacy against LM.
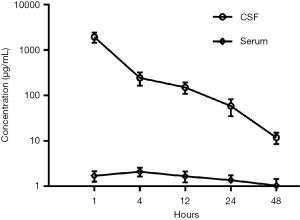
Regarding the optimal dose and administration mode of intrathecal chemotherapy, no consensus has yet been reached. Based on related animal trials and the standard use of intravenous administration (20,21), 30 mg of pemetrexed was given on days 1 and 8 every 3 weeks. The drug concentrations in CSF were measured before and after treatment. As shown in Figure 4, the pemetrexed level was clearly higher than that attained via intravenous delivery at this or a higher dosage (22), which further confirmed the validity of this therapeutic approach. However, this patient refused to try a higher therapeutic dose, so more cases and further studies are required to elucidate the optimum pemetrexed dose.
The complication rate of intraventricular reservoirs is low (23). No complications related to the Ommaya capsule (e.g., infection, tube misalignment and clogging, intracranial hematoma or CSF fistula) were found in this case. After the intrathecal treatment, the patient presented with a mild headache, nausea and vomiting, which were thought to be related to the changes in the CSF volume and gastrointestinal reactions to pemetrexed.
In addition to local intensive therapy, systemic antitumor treatment is also indispensable for LM treatment. The third-generation TKI osimertinib has attracted wide attention because of its standout CNS permeability and reliable antitumor activity (24), and it is the preferred choice for patients with LM from EGFR-mutant NSCLC (2,25). The case reported here was diagnosed LM at the standard dose (80 mg/day) of osimertinib; however, 160 mg daily osimertinib further prolonged her survival. This effect is probably because the higher osimertinib dose leads to a higher local concentration in the CNS. As a consequence, when osimertinib does not effectively control EGFR-mutated LM, a therapeutic approach consisting of local therapy and higher osimertinib doses is worth considering.
In summary, intrathecal pemetrexed via an Ommaya reservoir had a positive effect on LM from NSCLC. Additional clinical cases and further prospective studies are needed to assess the safety, effectiveness and the ideal combination of treatments.
Acknowledgments
Funding: This work was supported by funding from the Key Project of Nanjing Health Bureau (to ZY) (No. ZKX16031), the Healthcare Project of Nanjing Science and Technology Committee (to ZY) (No. 201715020), the Medical Key Science and Technology Development Project of Nanjing (to ZY) (No. ZKX18014), the Cadre Health Care Project of Jiangsu Province (to MH) (No. BJ18006) and the Cancer Research Funding of CSCO-Hausen (to ZY) (No. Y-HS2019-5).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-521). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reckamp KL. Targeted therapy for patients with metastatic non-small cell lung cancer. J Natl Compr Canc Netw 2018;16:601-4. [Crossref] [PubMed]
- Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncology 2018;19:e43-55. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev 2017;53:128-37. [Crossref] [PubMed]
- Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med 1975;293:161-6. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol 2010;22:627-35. [Crossref] [PubMed]
- Burch PA, Grossman SA, Reinhard CS. Spinal cord penetration of intrathecally administered cytarabine and methotrexate: a quantitative autoradiographic study. J Natl Cancer Inst 1988;80:1211-6. [Crossref] [PubMed]
- Wilson R, Osborne C, Halsey C. The use of Ommaya reservoirs to deliver central nervous system-directed chemotherapy in childhood acute lymphoblastic leukaemia. Paediatr Drugs 2018;20:293-301. [Crossref] [PubMed]
- Volkov AA, Filis AK, Vrionis FD. Surgical treatment for leptomeningeal disease. Cancer Control 2017;24:47-53. [Crossref] [PubMed]
- Montes de Oca Delgado M, Cacho Diaz B, Santos Zambrano J, et al. The comparative treatment of intraventricular chemotherapy by Ommaya reservoir vs. lumbar puncture in patients with leptomeningeal carcinomatosis. Front Oncol 2018;8:509. [Crossref] [PubMed]
- Roguski M, Rughani A, Lin CT, et al. Survival following Ommaya reservoir placement for neoplastic meningitis. J Clin Neurosci 2015;22:1467-72. [Crossref] [PubMed]
- Hitchins RN, Bell DR, Woods RL, et al. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol 1987;5:1655-62. [Crossref] [PubMed]
- Sandberg DI, Bilsky MH, Souweidane MM, et al. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery 2000;47:49-54; discussion 54-5. [PubMed]
- Lavrador JP, Simas N, Oliveira E, et al. Intratechal chemotherapy treatment through an Ommaya reservoir catheter for meningeal carcinomatosis: a single-centre experience. Acta Med Port 2016;29:456-60. [Crossref] [PubMed]
- Pardo-Moreno J, Fernandez C, Arroyo R, et al. Safety of intra-cerebrospinal fluid chemotherapy in onco-haematological patients: a retrospective analysis of 627 interventions. J Neurooncol 2015;125:351-8. [Crossref] [PubMed]
- Zairi F, Le Rhun E, Bertrand N, et al. Complications related to the use of an intraventricular access device for the treatment of leptomeningeal metastases from solid tumor: a single centre experience in 112 patients. J Neurooncol 2015;124:317-23. [Crossref] [PubMed]
- Gwak HS, Joo J, Kim S, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol 2013;8:599-605. [Crossref] [PubMed]
- Stapleton SL, Reid JM, Thompson PA, et al. Plasma and cerebrospinal fluid pharmacokinetics of pemetrexed after intravenous administration in non-human primates. Cancer Chemother Pharmacol 2007;59:461-6. [Crossref] [PubMed]
- Sun JM, Nam MH, Chung JY, et al. Safety and pharmacokinetics of intrathecal administration of pemetrexed in rats. Cancer Chemother Pharmacol 2011;68:531-8. [Crossref] [PubMed]
- Pan Z, Yang G, Cui J, et al. A pilot phase 1 study of intrathecal pemetrexed for refractory leptomeningeal metastases from non-small-cell lung cancer. Front Oncol 2019;9:838. [Crossref] [PubMed]
- Kumthekar P, Grimm SA, Avram MJ, et al. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J Neurooncol 2013;112:247-55. [Crossref] [PubMed]
- Wang A, Tenner MS, Tobias ME, et al. A novel approach using electromagnetic neuronavigation and a flexible neuroendoscope for placement of Ommaya reservoirs. World Neurosurg 2016;96:195-201. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]



