
The poor prognosis of lower-inner quadrant breast cancer in patients who received neoadjuvant chemotherapy
Introduction
Breast cancer is still a threat to women’s health despite marked advances in cancer research. Neoadjuvant chemotherapy (NAC) refers to a choice of primary therapeutic methods used before surgery to treat breast carcinoma tumors. NAC has commonly been applied to treat inoperable breast carcinoma. Currently, NAC’s role in treating operable breast carcinoma is recognized, as it can be used in all breast carcinoma cases that require chemotherapy. Thus far, several clinicopathological factors, including axillary lymph node (ALN) metastasis, tumor location, number of lymph nodes at first diagnosis, and molecular subtype, have already been shown to be associated with the prognosis associated with NAC by a large number of retrospective studies. Regarding tumor location, in addition to being the less frequent site for breast cancer occurrence, inner-quadrant cancer is often characterized by poor prognosis and shorter breast cancer-specific survival (BCSS) and overall survival (OS). Nevertheless, the study conducted by Chang et al. (1) was the only clinical study to explore the prognostic value of tumor size on the survival of breast cancer patients who received NAC treatment. Moreover, the clinical trial conducted by Jiqiao Yang revealed that tumors in the lower-inner zone (LIZ) had a markedly poor prognosis in terms of disease-free survival (DFS) in patients in the NAC subgroup.
Many clinical trials related to the clinical significance of tumor site have shed light on applying postmastectomy radiation therapy (PMRT) or internal mammary node radiotherapy (IMN-RT) more specifically and identifying the high-risk groups. Moreover, there is debate about how PMRT for NAC patients is to be applied. In the research of Le Scodan, Shim, and colleagues, patients with clinical stage II-III disease who achieve ypN0 after receiving NAC will be at a lower risk of recurrence without treatment with PMRT. According to recent publications, administering PMRT to ypN2–3 rather than ypN0–1 women is also related to enhanced locoregional recurrence-free survival (LRRFS) and OS. The purpose of our present study was to explore the prognostic implication of tumor location on the survival of clinical II–III patients treated with NAC and to explore further the effect of PMRT on tumors in different quadrants.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1140).
Methods
The experimental protocol was set up according to the ethical guidelines of the Declaration of Helsinki (as revised in 2013) and was approved by the Human Ethics Committee of Harbin Medical University. Written informed consent was obtained from the participants or their guardians.
The first cohort in this study was composed of 676 patients who were diagnosed with breast carcinoma from 2010 to 2014 and later received NAC. The inclusion criteria included the following: (I) underwent normal radical or optimized radical mastectomy; (II) had no history of other malignancy; and (III) did not receive preoperative radiotherapy (RT) or postoperative chemotherapy. All the patients had standard bone marrow and intact hepatic, cardiac, and renal function. Patients with multicentric tumors were not included in our retrospective cohort; those with unknown tumor locations were also excluded. The regimens for chemotherapy were (I) docetaxel and cyclophosphamide (TC) (6 cycles) (n=96, 14.2%), (II) doxorubicin and docetaxel (AT) (6 cycles) (n=270, 39.9%), and (III) docetaxel, doxorubicin and cyclophosphamide (TAC) (6 cycles) (n=310, 45.9%). The NAC regimens were as follows: AT, doxorubicin (60 mg/m2) and docetaxel (75 mg/m2) by intravenous infusion for three weeks for a total of four to six cycles; TC, docetaxel: (75 mg/m2) and cyclophosphamide (600 mg/m2) by venous infusion for three weeks for a total of 4–6 cycles; and TAC, docetaxel (75 mg/m2), doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) by intravenous infusion for three weeks for a total of four to six cycles. Most patients in the first clinical stages of IIA, IIB, and IIIA received AT and TC therapy. The TAC regimen was primarily prescribed to the patients with stage IIIB or IIIC cancer. After receiving NAC, all patients underwent surgery with either modified radical mastectomy (MRM) or breast-conserving surgery (BCS) with ALN dissection.
All cases received preoperative examination, which included mammography and ultrasonography on breast and axilla. After undergoing surgery, the patients underwent radiation or endocrine therapy. RT was performed in different manners according to the surgical approach, tumor location, response to NAC, among many others. In our study, most of the clinical-stage II patients who achieved the pathological complete response (pCR) or ypN0 before MRM did not receive any form of PMRT. PMRT was strongly recommended to the patients with first clinical-stage IIIB-C regardless of the pathologic extent of disease before the operation and to patients with clinical stage II with ypN+. For the clinical-stage IIIa patients, PMRT was applied according to the initial N stages, luminal subtype, T stage, age, and the patient’s desire to undergo the treatment. The patients who had undergone BCS were all treated with a 50.4 Gy dose in total at 1.8 Gy per fraction to the entire breast for five fractions each week; subsequently, the patients received an electron boost (10 Gy) to the target area (tumor bed). For the patients who underwent MRM, the target area, including the local or nearby lymph node site and the chest wall, and the identical dose method was employed. IMN-RT was performed on patients based on clinical or pathological characteristics. IMN-RT could be applied to treat inner-quadrant tumors that might be progressive. According to the National Comprehensive Cancer Network (NCCN) guidelines, regarding adjuvant hormonal therapy, premenopausal females were administered tamoxifen for five years, and postmenopausal females were treated with an aromatase inhibitor or with sequential tamoxifen treatment followed by treatment with an aromatase inhibitor.
Evaluation of clinical and pathological results
Patients were further subdivided into five quadrants according to their primary tumor sites after the first physical and imaging examination. It was found that a vast majority of patients had a primary tumor located in the upper-outer quadrant (54%) and upper-inner quadrant (19.8%), whereas a minority of patients had a primary tumor in the lower-inner (13.8%), central (2.5%) and lower-outer quadrants (9.8%). A larger cohort of patients would be required to improve the quality of our clinical study to address statistical discrepancies. Finally, we excluded the minority and split the cohorts into only three groups according to tumor site: outer quadrant, upper-inner quadrant, and lower-inner/central quadrant.
The prognostic implications of pathologic findings and treatment features were assessed in addition to the tumor location. Among the pathological and clinical variables, primary tumor size, lymph node status, human epidermal growth factor receptor 2 (HER-2) status, etc. were assessed as independent variables. Among the treatment characteristics, the types of surgery, IMN-RT, and hormonal therapy were assessed. Furthermore, pCR was defined as no residual invasive tumor in the breast. The tumors with residual intraductal carcinoma were also included in the pCR group.
By Pearson chi-square test, the features of the lower-inner/central, upper-outer, and upper-inner quadrant groups were compared. Chi-square test (Fisher’s exact test) was performed to compare the pCR rate between the two-quadrant groups. Local recurrence stood for a kind of recurrence that occurred in the ipsilateral breast or the chest wall. Regional recurrence is always defined as a relapse found in the ipsilateral axillary, supraclavicular, and/or internal mammary node (IMN) regions. Recurrence in the contralateral breast, liver, lung, or other distant organs was considered to be distant metastasis. DFS refers to the period between the date of any disease recurrence and the date of the first NAC. OS refers to the time from the date of NAC to the date of death. By using the Kaplan-Meier method, we compared the DFS, locoregional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), and OS between the distinct groups. The log-rank test was performed for univariate analysis. Using the Cox proportional hazards regression model, the variables that were statistically significant in the univariate analysis were included in the multivariate analysis. All P values <0.05 were defined to show statistical significance. For subgroup analysis, the P value was corrected according to the Bonferroni correction for reiterated measurement (a=0.05/n). All statistical analyses were conducted using SPSS version 22.0 software.
Results
Here, 676 patients aged 53 (23–70) years, on average, received NAC. To be specific, 110 patients had lower-inner/central quadrant tumors, 134 had upper-inner quadrant tumors, and 432 cases had outer quadrant tumors. Table 1 lists patient and treatment features according to tumor sites.
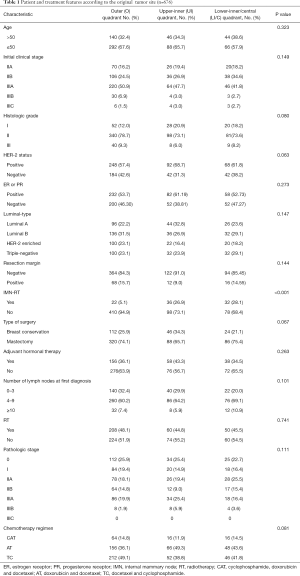
Full table
Most patients received the whole course of chemotherapy (6 cycles of AT, TC, and TAC). NAC resulted in no notable toxicity that could have led to dose regulation or the delay of treatment. The median interval time was 25 (8–71) days from the last day of NAC to surgery. A total of 494 patients underwent MRM, and 182 underwent BCS. In total, 18 patients (14.3%) showed positive or close resection margins, while 43 underwent added total mastectomy for diffuse tumor infiltration. Also, added surgery was avoided according to the institutional policy. Most of the PMRT was provided to ypN+ patients. IMN-RT was highly recommended for the inner quadrant patients with ypN+ after NAC. In total, 252 and 308 patients received adjuvant hormonal therapy and adjuvant RT, respectively. All patients who were subjected to RT after MRM received RT locally as well as to their chest walls (246 49.8%). Of the patients who underwent BCS, 64 (45.7%) underwent whole breast RT only, and 76 (55.3%) received regional RT. Last, only a small proportion of the patients who underwent MRM (10.5%) and those who had received BCS (8.8%) underwent IMN-RT.
The median follow-up period was 87 (21–106) months. One hundred twenty-eight patients (128/676, 18.9%) achieved pCR after NAC. In the comparison between the clinical stage before NAC and the postoperative pathological stage, 470 patients (69.5%) downstaged, 144 patients (21.3%) remained the same as before, and 62 (9.2%) experienced disease progression. For all patients, the 5-year DFS, LRRFS, DMFS and OS rates reached 77.8%, 93.2%, 83.7%, and 88.5%, respectively.
Recurrence analysis
A total of 169 out of 676 patients (24.1%) experienced total treatment failure. According to the original tumor site, the three groups were similar in locoregional control rate (outer quadrant, 25/432 5.8%; upper inner quadrant, 9/134 6.7%; inner/central quadrant, 6/110 14.0%) (Table 2). The original pattern of failure in all patient groups was distant metastasis (126/676 18.6%). There were twenty cases of contralateral breast recurrence (outer quadrant, 8; upper inner quadrant, 6; lower-inner/central quadrant, 6), which was distant metastasis.

Full table
Univariate analysis
Tumor location (P=0.001), initial clinical stage (P<0.001), nuclear grade (P<0.001), luminal-type (P=0.002) and pathological stage (P=0.012) were found to be the factors affecting DFS (Figure 1A). Meanwhile, LRRFS was influenced by luminal-type (P<0.001), HER-2 status (P=0.002) (Figure 1B), type of surgery (P=0.003), initial clinical stage (P=0.015) and pathological stage (P=0.002). Nevertheless, tumor location (P<0.001), nuclear grade (P<0.001), initial clinical stage (P<0.001), age (P=0.044) and type of surgery (P=0.026) were found to be the factors affecting DMFS (Figure 1C, Table 3). The factors affecting OS included tumor location (P=0.002), nuclear grade (P<0.001), initial clinical stage (P=0.006) luminal type (P=0.002) and number of lymph nodes at first diagnosis (P<0.001) (Figure 1D). Tumor location functioned as a significant prognostic factor for DFS, DMFS and OS. Though the data were statistically nonsignificant, we observed that IMN-RT improved DFS (80.3% vs. 73.6%; 5-year, P=0.142), LRRFS (96.5% vs. 93.4% 5-year, P=0.179), DMFS (80.3% vs. 80.5%; 5-year, P=0.996) and OS (85.9% vs. 89.1%; P=0.494). When stratified into subdivided groups, the patients with tumors in the LIZ had a noticeably poor prognosis in terms of DFS, DMFS and OS among patients in the subgroups of triple-negative status (P<0.001, a=0.025; P<0.001, a=0.025; P<0.001, a=0.025), HER-2 status (P=0.017, a=0.025; P=0.017, a=0.025; P<0.002, a=0.025) and those who did not receive RT (P<0.001, a=0.025; P<0.001, a=0.025; P=0.010, a=0.025) (Table S1).
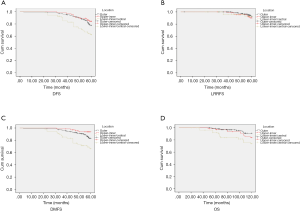
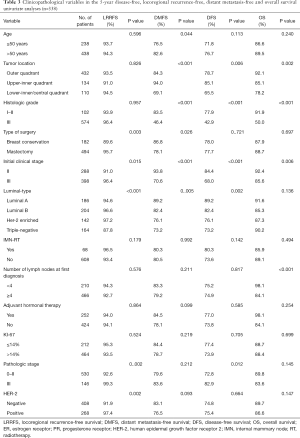
Full table
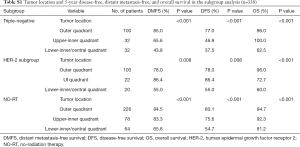
Full table
Multivariate analysis
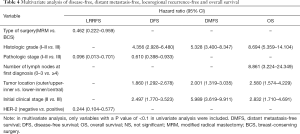
Multivariate analysis was applied using the variables that showed statistical significance in the univariate analysis in the Cox proportional hazards regression model (Table 4). In the analysis, initial clinical grade (P<0.001), tumor location (P=0.001), nuclear grade (P<0.001), and pathologic stage (P=0.023) were recognized to be independent factors influencing DFS. Nuclear grade, initial clinical stage, and tumor location were significant factors affecting DMFS (P<0.001, P<0.001, P<0.001, respectively). Her2, type of surgery, and pathologic stage were significant factors affecting LRRFS (P=0.002, P=0.029, and P=0.021, respectively). In the multivariate analysis, tumor location, nuclear grade, initial clinical stage, and the number of lymph nodes at the first diagnosis were identified to be significant factors affecting OS (P=0.002, P<0.001, P=0.006 and P<0.001, respectively).
Full table
Discussion
According to the results of the retrospective study by Chang et al. (1), tumor site affects the survival of breast carcinoma patients who received NAC for the first time. Unlike the earlier clinical trials, our study primarily focused on three significant quadrants in our NAC setting. According to our data, the lower-inner/central quadrant tumors had a worse prognosis (including prognosis in terms of DFS, DMFS, and OS) than the outer and upper-inner quadrant tumors.
Tumor location has been one of the most controversial prognostic indicators for breast cancer, and it will be critical to explore whether tumor location may also be critical in the prediction of the clinical prognosis of breast cancer patients who underwent NAC. The prognostic implication of tumor location has been explored in numerous clinical trials with inconsistent results. A SEER population-based clinical retrospective study performed by Jing Bao and his partner (2) reported that patients with tumors in the lower-outer quadrant on either side of the left central quadrant were at increased risk of relapse based on a cohort of 305,443 patients. These clinical retrospective studies (3-5) revealed that the tumor quadrant had a specialized ability to assess the prognosis of early breast carcinoma. However, in the study by Hwang KT, for lymph node-negative patients, the lower-inner quadrant was associated with worse OS than other primary tumor sites in the subgroup of patients who had not been treated with chemotherapy.
In contrast, concerning the subgroup of patients who had been treated with chemotherapy, such a phenomenon was not observed (6). Based on the mentioned existing studies, the IMNs can be used to explain the poor prognosis of patients with inner and central quadrant breast cancer from a mechanistic perspective. IMNs are a type of “first station” lymph node of breast cancer lymphatic drainage, namely, one of the critical metastatic pathways of inner and central quadrant breast cancer (7). Most of the current studies support the concept that hidden IMN contributes to the poor prognosis of inner quadrant tumors. It has been extensively reported that patients with IMN metastases had a worse prognosis than patients who did not, regardless of the axillary status (8-10). The retrospective study conducted by Lukesova et al. found that lower-outer, lower-inner, and upper-inner quadrant tumors were more prone to be characterized as IMN enrollment (11). The study conducted by D R Byrd showed that drainage to IMN following quadrant location of the lesion was as follows: UOQ, 10%; LOQ, 27%; UIQ, 17%; LIQ, 25%; and central, 29%. They also concluded that tumors in all quadrants might drain to IMNs; although, the drainage is significantly more common from inner quadrants than UOQ (12). From the above two studies, we noticed that the LIQ has a significantly lower rate of drainage than all other quadrants. Our current knowledge on the prognosis of the tumor location states that tumors in the lower-inner/central quadrant had a noticeably poor prognosis in terms of DFS, DMFS, and OS. The mechanisms can be explained by the high incidence of the hidden IMN metastasis and the low rate of IMN-RT for the lower-inner and central quadrant. The deficiency of a proper tracing method made us fail to detect the IMN recurrence after surgery and radiation therapy. It is worth mentioning that the upper-inner quadrant did not share the best or the worst prognosis. The mechanism behind it may be a small amount, which may lead to a biased result.
On the other hand, the hidden IMN originated from the upper-inner quadrant may be affected by radiotherapy on the post-mastectomy chest wall or the upper and lower clavicular regions. These clinical studies led to the development of explorations of means of diagnosing techniques for the IMN chain. However, due to the location and the small size of it, inner mammary lymph nodes are extremely difficult to be detected.
The internal mammary lymph node (IMLN) chain refers to a wide range of lymph node channels that have significant individual differences, and the management of IMLNs has always been debatable. Likewise, there is a controversy regarding the risk versus benefit of including the internal mammary chain in the radiation field: Courdi et al. reported that IMN-RT for node-negative tumors was associated with the promotion of OS and CSS among patients with lesions located in the inner and central quadrants (12). A larger-scale retrospective clinical study with a 12-year follow-up revealed that IMN-RT significantly prolonged DFS in breast cancer patients after patients had undergone mastectomy (13). However, the study conducted by Fowble et al. did not find a noticeable clinic advantage associated with IMN irradiation in terms of DMFS or cause-specific survival among all patients, especially among patients with positive axillary nodes and lesions located at the inner quadrant (14). In our clinical practice, no consensus has been reached concerning the RT field after NAC and operation. These results revealed that the RT field always was heterogeneously applied among patients at even rates among those with identical clinical stages. Most of the patients with first clinical-stage II–III breast cancer tumors (73.9%) who had achieved complete nodal response and pCR to NAC after mastectomy in our cohort received no adjuvant RT. For the ypN+ patients after NAC, IMN-RT was highly recommended for the ones with inner quadrant tumors.
Nevertheless, the implementation of IMN-RT among ypN+ patients was often ignored after the adequate dosage and duration of NAC, operation, and post-surgery RT were performed; as a consequence, the metastasis of IMN might not be detected or treated promptly. Furthermore, our subgroup analysis revealed that lower-inner quadrant tumors maintained a significantly worse prognosis in terms of DFS, DMFS, and OS in cohorts of patients who did not receive RT, suggesting that the monitoring of IMLN was often ignored before or after treatment in the clinical setting. For fear of the cardiac toxicity, the majority (86.7%) of pN+ patients with primary tumors at inner sites refused IMN-RT. Only approximately 7.6% of inner quadrant patients with pN+ after operation accepted RT that included the IMLNs. These skewed distributions and the interaction of treatment characteristics might have worsened the outcomes associated with tumors in the lower-inner/central quadrant. The critical risk factor for recurrence is still unclear: the clinical stage at the time of presentation before the initiation of NAC or the residual pathologic disease burden at the time of surgery after NAC. As we proposed, the sign for RT should also be based on the primary tumor site. Given the prognostic implication of nodal positivity in the IMLN chain, the reconsideration of the surgical and RT approach for these nodes is reasonable, especially for patients with a tumor in the inner quadrant.
More advanced imaging techniques should be employed for the detection of IMN before and after NAC. Nevertheless, the definition of nodal pCR is evaluated based on the pathological state of the ALNs but ignores the pathological state of the IMNs. Accordingly, considerable studies have explored magnetic resonance imaging (MRI), positron emission tomography-computed tomography (PET-CT), sentinel node biopsy, and other methods for their ability to make a better diagnosis of IMN, with few tools currently available to assist clinicians (15-17). The benefits of the exploration of diagnostic methods based on the IMN chain in breast carcinoma patients are limited and are likely to be refused by patients due to the risks associated with the complications (17). Surgical techniques have also been developed for inner-quadrant breast cancer. Improving the clinical effect of surgical approaches for inner-quadrant breast cancer will require more insights into the factors that affect the thoracic cavity (18-20).
Regarding NAC, though numerous in-depth studies have discussed the additional contributions of RT for IMN, none have paid attention to the relationship between chemotherapy and the inner quadrant, which makes it challenging to interpret the results. A majority of our patients completed the whole course in our trial, and more advanced NAC (AT, CAT) did not result in improved DFS or OS in lower-inner/central quadrant breast cancer patients. These results suggested that modulation of RT or surgery targeting the IMN may be necessary for the design of therapy for inner-quadrant breast cancer. In our clinical practice, a small portion of patients with clinical stage IIIB or IIIC preferred the treatments with few side effects instead of the ones with the best efficacy; they chose the relatively milder chemotherapy regimens (AT or TC). This skewed distribution might have weakened the impact of the NAC on the inner-quadrant tumors (21). Nevertheless, data from our research is insufficient to confirm the view. Perhaps improving the long-term clinical effect of NAC will require a deeper understanding of the doses and regimens that impact inner-quadrant tumors and IMN, rather than prescribing chemotherapy regimens based on the clinical stage at first diagnosis.
This study has some limitations. Both the preoperative and postoperative assessment of IMN involvement is critical for showing the prognosis of the disease. However, in practice, ultrasound was most frequently used by our patients, indicating that most of the IMNs were not examined or routinely treated. The latest NCCN clinical guidelines (22) strongly recommended regional nodal RT for patients with positive lymph nodes after NAC. More than 63.03% of our patients had more than 4 ALNs at first diagnosis, and a majority of them received adjuvant RT. Multivariate analysis suggested that in contrast to no ALN involvement, patients with 1–3 ALNs involved and even ≥4 ALNs involved displayed a noticeably increased risk of IMN metastasis (23). However, only approximately 65.9% of the patients with ≥4 lymph nodes at first diagnosis in our cohort received PMRT. Most importantly, few of them received IMN-RT.
Moreover, Clinical stage II–IIIA patients accounted for the most substantial number of patients in our trial (626 patients). Stage IIIB and IIIC breast cancer typically have a high rate of relapse due to the late stage. Enrolling more IIIB and IIIC patients may need to be considered in the design of a clinical trial to illustrate better the prognosis based on the tumor location. Regarding the subgroup aspects, the IMN-RT group was identified to have only a slight survival benefit in comparison with the non-IMN-RT group in patients with lower-inner/central quadrant breast cancer (no relevant data presented). It is known that HER-2-enriched and triple-negative subgroup patients have an elevated risk of recurrence. However, the IMN-RT group did not have better survival than the non-IMN-RT group among patients with cancer in the lower-inner/central quadrants (55.56% vs. 52.94%) in the HER-2-enriched and triple-negative subgroups. More data are needed to prove the clinical benefits of IMN-RT.
Moreover, regarding the luminal subtype (luminal A and luminal B), characterized as being associated with a lower risk for developing a recurrent disease, the phenomenon of a higher risk of recurrence no longer existed (data not are shown) for the lower-inner quadrant. Due to the patients in our retrospective study being mainly patients in the first cohorts who accepted NAC, most of the HER-2 positive cases neither received the neoadjuvant anti-HER-2 therapy nor completed the whole course of follow-up anti-HER2-target treatment. The difficulty of the follow-up period worsened the situation. Furthermore, it may have led to confusion in the assessment of the benefit of IMN-RT.
From the current literature, how the inner quadrant microenvironment sets the stage for probable tumor formation and enhances the metastatic potential is still unclear. It was already known that breast cancer development in the outer quadrants is much more frequent than that in the inner quadrants, and the investigation of the association between the quadrant site of tumors and quadrant density will be worth conducting (24). Two studies have evaluated breast density in a range of quadrants, but the results were inconsistent with the incidence of cancer (25,26). Other clinical trials have suggested that the prognostic value of the inner-quadrant location is evident in certain luminal types, whereas the findings have been inconsistent among different clinical trials (27,28). Tumorigenesis, the development mechanisms, and the microenvironment of inner-quadrant breast cancer may be complex and should be explored in subsequent studies.
Conclusions
In conclusion, despite different NAC regimens, the lower-inner/central tumor location in breast carcinoma was associated with lower DFS, DMFS, LRRFS, and OS than outer and upper inner quadrant tumors. More aggressive NAC with IMN-RT might be needed to address the poor results related to inner-quadrant participation in breast carcinoma. The potential mechanism underlying the unfavourability of inner tumors should be explored. It is difficult to use the site of the primary tumor as a prognostic criterion until the mechanism is clarified.
Acknowledgments
We appreciate Qingyuan Zhang for revising the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1140
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1140
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1140). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experimental protocol was set up according to the ethical guidelines of the Declaration of Helsinki (as revised in 2013) and was approved by the Human Ethics Committee of Harbin Medical University. Written informed consent was obtained from the participants or their guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang JH, Jeon W, Kim K, et al. Prognostic Significance of Inner Quadrant Involvement in Breast Cancer Treated with Neoadjuvant Chemotherapy. J Breast Cancer 2016;19:394-401. [Crossref] [PubMed]
- Bao J, Yu KD, Jiang YZ, et al. The Effect of Laterality and Primary Tumor Site on Cancer-Specific Mortality in Breast Cancer: A SEER Population-Based Study. PLoS One 2014;9:e94815. [Crossref] [PubMed]
- Niu S, Wen G, Ren Y, et al. Predictive Value of Primary Tumor Site for Loco-regional Recurrence in Early Breast Cancer Patients with One to Three Positive Axillary Lymphadenophy. J Cancer 2017;8:2394-400. [Crossref] [PubMed]
- Yang J, Tang S, Zhou Y, et al. Prognostic implication of the primary tumor location in early-stage breast cancer: focus on lower inner zone. Breast Cancer 2018;25:100-7. [Crossref] [PubMed]
- Gaffney DK, Tsodikov A, Wiggins CL. Diminished survival in patients with inner versus outer quadrant breast cancers. J Clin Oncol 2003;21:467-72. [Crossref] [PubMed]
- Hwang KT, Kim J, Kim EK, et al. Poor Prognosis of Lower Inner Quadrant in Lymph Node-negative Breast Cancer Patients Who Received No Chemotherapy: A Study Based on Nationwide Korean Breast CancerRegistry Database. Clin Breast Cancer 2017;17:e169-84. [Crossref] [PubMed]
- Bi Z, Chen P, Liu J, et al. Internal Mammary Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer. J Breast Cancer 2018;21:442-6. [Crossref] [PubMed]
- Kroman N, Wohlfahrt J, Mouridsen HT, et al. Influence of tumor location on breast cancer prognosis. Int J Cancer 2003;105:542-5. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects ofradiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Kroman N, Wohlfahrt J, Mouridsen HT, et al. Influence of tumor location on breast cancer prognosis. Int J Cancer 2003;105:542-5. [Crossref] [PubMed]
- Lukesova L, Vrana D, Svach I, et al. Prognostic Influence of Internal Mammary Node Drainage in Patients with Early-stage Breast Cancer. Anticancer Res 2016;36:6641-6. [Crossref] [PubMed]
- Courdi A, Chamorey E, Ferrero JM, et al. Influence of internal mammary node irradiation on long-term outcome and contralateral breast cancer incidence in node-negative breast cancer patients. Radiother Oncol 2013;108:259-65. [Crossref] [PubMed]
- Chang JS, Park W, Kim YB, et al. Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat Oncol Biol Phys 2013;86:867-72. [Crossref] [PubMed]
- Fowble B, Hanlon A, Freedman G, et al. Internal mammary node irradiation neither decreases distant metastases nor improves survival in stage I and II breast cancer. Int J Radiat Oncol Biol Phys 2000;47:883-94. [Crossref] [PubMed]
- Bi Zhao, Zheng Wei-Zhen, Qiu Heng, et al. Internal mammary sentinel lymph node biopsy with modified injection technique. Medicine (Baltimore) 2017;96:e9466. [Crossref] [PubMed]
- Jochelson MS, Lebron L, Jacobs SS, et al. Detection of Internal Mammary Adenopathy in Patients With Breast Cancer by PET/CT and MRI. AJR Am J Roentgenol 2015;205:899-904. [Crossref] [PubMed]
- Tan C, Caragata R, Bennett I, et al. Is Sentinel Node Biopsy of the Internal Mammary Lymph Nodes Relevant in the Management of Breast Cancer? Breast J 2017;23:410-4. [Crossref] [PubMed]
- Lin J, Chen DR, Wang YF, et al. Oncoplastic Surgery for Upper/Upper Inner Quadrant Breast Cancer. PLoS One 2016;11:e0168434. [Crossref] [PubMed]
- Lee S, Lee J, Jung Y, Bae Y. Oncoplastic surgery for inner quadrant breast cancer: fish-hook incision rotation flap. ANZ J Surg 2017;87:E129-33. [Crossref] [PubMed]
- Lin J, Chen DR, Wang YF, et al. Oncoplastic Surgery for Upper/Upper Inner Quadrant Breast Cancer. PLoS One 2016;11:e0168434. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Breast Cancer (2019 Version 1) [DB/OL]. Available online: http://www.nccn.org.
- Qi XW, Du JZ, Tang P, et al. Clinical significance of internal mammary lymph node metastasis for breast cancer: Analysis of 337 breast cancer patients. Surg Oncol 2018;27:185-91. [Crossref] [PubMed]
- Chan S, Chen JH, Li S, et al. Evaluation of the association between quantitative mammographic density and breast cancer occurred in different quadrants. BMC Cancer 2017;17:274. [Crossref] [PubMed]
- Vachon CM, Brandt KR, Ghosh K, et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomarkers Prev 2007;16:43-9. [Crossref] [PubMed]
- Ursin G, Hovanessian-Larsen L, Parisky YR, et al. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res 2005;7:R605-8. [Crossref] [PubMed]
- Zhu J, Jiao D, Guo X, et al. Predictive factors and prognostic value of pathologic complete response of ipsilateral supraclavicular lymph nodes in breast cancer after neoadjuvant chemotherapy. Ann Transl Med 2019;7:666. [Crossref] [PubMed]
- Siotos C, McColl M, Psoter K, et al. Tumor Site and Breast Cancer Prognosis. Clin Breast Cancer 2018;18:e1045-52. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


