
Sophocarpine prevents cigarette smoke-induced restenosis in rat carotid arteries after angioplasty
Introduction
Nearly 20% of the world’s population are current smokers (1). Cigarette smoke is one of the most important sources of chemical toxicity in human beings (2,3). Cigarette smoking is also the main underlying cause of atherosclerotic occlusive disease, including stroke, heart attack and peripheral arterial disease (4,5). Increasing evidence has shown that cigarette smoking-related artery diseases can be explained by the inflammatory reaction in the vascular wall, such as the infiltration of inflammatory cells and secretion of inflammatory mediators (6,7). Clinically, interventional angioplasty is a widely used and less invasive treatment for atherosclerotic occlusive disease. However, the long-term patency of the target artery can not be maintained easily, due to the occurrence of restenosis. Moreover, a higher incidence of restenosis can be observed among smokers. As a result, smokers suffering from atherosclerotic occlusive disease may receive interventional angioplasty more frequent compared with non-smokers. In addition, previous studies have demonstrated that cigarette smoking-induced inflammatory reaction is associated with higher rates of restenosis (8). Furthermore, the increased expression levels of inflammatory mediators, such as C-reactive protein, interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), can be found in the serum of smokers (9,10). Therefore, it is necessary to exploit a drug for alleviating inflammatory response and preventing restenosis in patients who continued to smoke after interventional angioplasty.
Enough evidences indicate that the p38 pathway plays a major role in the inflammatory responses (11,12). The p38 kinases can be activated by dual kinases called the MAP kinase kinases (MKKs). Of all the MKKs, MKK3 and MKK6 are the two main MAPKKs that can activate p38 by phosphorylation at Thr180 and Tyr182 (13). Accumulating evidence suggests that the MKKs/p38 MAPK pathway is involved in the expression of many proinflammatory mediators such as IL-1β, IL-12, TNF-α and PGE2 (14). This pathway can be activated in response to stress and inflammatory stimuli, such as heat shock, lipopolysaccharide, inflammatory cytokines, osmotic shock, ultraviolet irradiation, and growth factors (15). Researches showed that the MKKs/p38 MAPK pathway was related to atherosclerosis, disfunction of endothelial cells, hypertrophy of smooth muscle cells and aortic valve sclerosis (16). That implies the MKKs/p38 MAPK pathway can be used as a pharmacological target to treat vascular diseases.
Sophora flavescens, a traditional Chinese herb, is used to treat a wide variety of diseases since ancient times. Sophocarpine is one of the typical sophora alkaloids extracted from Sophora flavescens (17). Sophora alkaloids share many common molecular structures and exert various therapeutic effects including anti-oxidation, anti-inflammation, anti-tumor, antivirus and immune regulation (18-20). Sophora alopecuroides Linn. (Kudouzi) can also be used to treat inflammation, pain, edema and fever (21). A previous study has revealed that sophocarpine inhibits the expression of IL-6 and TNF-α in murine macrophage cells and prevents cachexia-associated symptoms in mice bearing colon-26 adenocarcinoma (22). Furthermore, sophocarpine can suppress inflammatory mediator production, neutrophil infiltration and myeloperoxidase activity (23). More importantly, sophocarpine injection has been used to treat viral myocarditis in clinical trials (21). Based on the findings of these studies, we speculated that sophocarpine might exhibit protective role in cigarette smoke-induced restenosis after interventional angioplasty.
In this study, we assessed the protective effects of sophocarpine on neointima formation and inflammatory mediator production in the balloon-injured carotid arteries of rats exposed to cigarette smoke. In addition, the therapeutic applicability of sophocarpine for preventing restenosis in smokers after interventional angioplasty was evaluated. Moreover, to stimulate the interventional angioplasty in smokers, we used the balloon to injure the carotid endothelium of rats after exposure to cigarette smoke. To the best of our knowledge, this study is the first to investigate the protective effects of sophocarpine on cigarette smoke-induced restenosis after interventional angioplasty. We present the following article in accordance with the 15-ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-19-568).
Methods
Animals
This study complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and the Animal Management Rule of the Chinese Ministry of Health, and was approved by the Animal Care Committee of Peking Union Medical College (JS-863). A total of 15 male Sprague-Dawley rats (mean weight: 350 g) were obtained for the experiments. All rats were randomly divided into 3 groups (n=5 in each group): Group A (control group; the rats were subjected to air exposure, saline injection and balloon injury), Group B (smoking group; the rats were subjected to cigarette smoking, saline injection and balloon injury), and Group C [smoking + sophocarpine group; the rats were subjected to cigarette smoking, sophocarpine (SML2422; Sigma-Aldrich, St. Louis, MO, USA) injection and balloon injury].
The animals in Group B and C were exposed to the smoke of 10 cigarettes (1.2 mg nicotine and 13 mg tar per cigarette) daily for seven days based on the modified protocol (24), while those in Group A were exposed to air. Meanwhile, the animals in Group C were intraperitoneally administered with 40 mg/kg sophocarpine (25) each day during cigarette smoking, while those in Group A and B were injected with equivalent volumes of saline solution. After cigarette exposure for 7 days, a well-established carotid artery balloon injury approach was performed on all the three groups (26). The steps for smoke exposure and drug injection were repeated according to the aforementioned methods. At day 14 after the injury, the animals were intraperitoneally anaesthetized with pentobarbital sodium (Sunbiotech, Beijing, China). Finally, the contralateral and injured carotid arteries were excised, and stored in 4% paraformaldehyde at liquid nitrogen temperatures until further analysis.
Histological analysis
The injured arteries were prepared in xylene and graded alcohols, followed by paraffin embedding and serial sectioning at a 4-µm thickness. Subsequently, all sections were subjected to Verhoeff-Van Gieson staining by using an Elastic Stain Kit (HT25A; Sigma-Aldrich, St. Louis, MO, USA) as per the manufacturer’s protocol, After staining, the slides were examined using a DMI4000 B microscope (Leica Microsystems, Wetzlar, Germany), and all images were captured at 100× magnification. The neointima and media areas of the injured arteries were quantitatively measured with Image-Pro Plus version 6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA), and the ratio of carotid neointima/media area was then calculated.
Western blotting
Briefly, the injured arteries stored at liquid nitrogen temperatures were homogenized with 600 µL cell lysis buffer [62.5 mM Tris-HCl (AMRESCO, Solon, OH, USA), 2% SDS (Biotopped Co., Ltd., Beijing, China), 10% glycerol (AMRESCO, Solon, OH, USA); pH 6.8] in a mortar. Subsequently, the lysates were heated at 100 °C for 10 minutes, and then centrifuged at 10,800 ×g for 15 minutes. After supernatant collection, the total content of protein was assessed by BCA method (Thermo Scientific, Inc., Waltham, MA, USA). The protein extracts (20 µg) were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Sunshine Biotechnology Co., Ltd, Nanjing, China), and then transferred onto polyvinylidene fluoride (PDVF) membrane (Merck Millipore, Bill card, MA, USA). The membrane was blocked with 5% skimmed milk (Applygen Technologies, Inc., Beijing, China) in TBST [20 mM Tris-HCl (AMRESCO, Solon, OH, USA), 137 mM NaCl (Beijing Chemical Works, Beijing, China), 0.05% Tween-20 (AMRESCO, Solon, OH, USA)] at room temperature for 1 hour. Subsequently, the blocked membranes were rinsed 3 times in TBST and incubated with the specific primary antibodies at 4 °C for overnight. Primary antibodies for MKK3 (1:1,000; #5674), MKK6 (1:1,000; #9264), phospho-MKK3/6 (1:1,000; #9231), p38 (1:1,000; #9212), phospho-p38 (1:1,000; #4511) and β-Actin (1:1,000; #4970) were supplied by Cell Signaling Technology (Danvers, MA, USA). After rinsing 3 times in TBST, the membranes were incubated with the secondary antibody (1:5,000; ZB-2301; Zhongshan Jinqiao Biotechnology, Beijing, China) at room temperature for 1 hour. Finally, the detection of antibody-antigen complexes was performed using an enhanced chemiluminescent reagent kit (TransGen Biotech, Beijing, China). The gray scale values of target proteins were quantified and measured using AlphaEase FC software version 4.0 (Alpha Innotech Corp., San Leandro, CA, USA).
Reverse transcription polymerase chain reaction (RT-PCR) assay
The mRNA levels of IL-1β and TNF-α were determined using RT-PCR assay. Total RNA was isolated from the carotid arterial homogenate using TRIzol reagent (Life Technologies, Carlsbad, CA, USA). cDNA was synthesized from 2 µg of total RNA using the GoScript Reverse Transcriptase (Promega, Madison, WI, USA). RT-PCR assays were conducted on a Veriti Thermal Cycler (Life Technologies, Carlsbad, CA, USA) using Takara Bio’s Tag polymerase (Otsu, Shiga, Japan). The primer sequences and amplification conditions of the target genes are summarized in Table 1. For validation of the linearity between pre- and post-amplified PCR products, RT-PCR testing was performed with various quantities of cDNA and different numbers of PCR cycles (range, 30–40 cycles).

Full table
Statistical analysis
All data were evaluated using SPSS software version 17.0 (Chicago, IL, USA), and interpreted as mean ± standard deviation (SD). one-way ANOVA was employed to compare the differences among multiple groups. P values of <0.05 were considered statistically significant.
Results
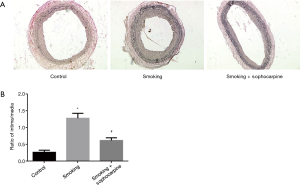
Cigarette smoke aggravates the stenosis of the lumen after balloon-induced carotid artery injury, and sophocarpine can prevent such process
The formation of neointimal lesion was found in rat carotid arteries following balloon injury, as revealed by histological analysis. Cigarette smoking aggravated neointima formation, but such process of stenosis could be retarded by sophocarpine. Figure 1A shows the representative cross-sections of the injured arteries among the three groups. The degree of lumen stenosis was quantified by the ratio of intima-to-media (I/M) area. As demonstrated in Figure 1B, sophocarpine could dramatically reduce the I/M ratio compared to smoking group (P<0.01). These results suggest that cigarette smoke may induce additional injury to the balloon-injured carotid arteries, and sophocarpine confers a protective effect during this process.
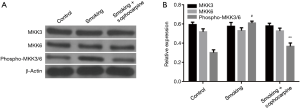
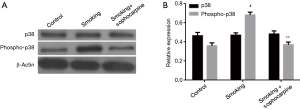
Cigarette smoke induces the expression levels of phos-MKK3/6 and phos-p38, and sophocarpine alleviates such effects
Western blotting was used to assess the protein levels of MKK3, MKK6, phos-MKK3/6, p38 and phos-p38. The results showed that cigarette smoke markedly elevated the levels of phos-MKK3/6 and phos-p38 in injured carotid arteries when compared to control group (Figures 2,3; P<0.01). To our expectation, sophocarpine remarkably down-regulated the expression levels of phos-MKK3/6 and phos-p38 (Figures 2,3; P<0.01). However, the protein levels of MKK3, MKK6 and p38 were not under the influence of cigarette smoke or sophocarpine (Figures 2,3). These findings revealed that cigarette smoke induced the phosphorylation levels of MKK3, MKK6 and p38, and treatment with sophocarpine could inhibit such effects.
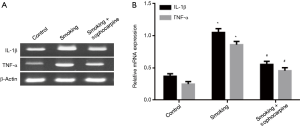
Sophocarpine can reduce the transcriptional levels of IL-1β and TNF-α induced by cigarette smoke
IL-1β and TNF-α are both critical mediators of inflammatory response, and their levels can indicate the degree of inflammatory reaction in tissue. In this study, RT-PCR assay was employed to assess the mRNA levels of IL-1β and TNF-α. As shown in Figure 4, the transcriptional levels of IL-1β and TNF-α were higher in smoking group compared to control group (P<0.01). Notably, sophocarpine could decrease the overexpression of IL-1β and TNF-α (Figure 4; P<0.01). These findings indicated that cigarette smoke could aggravate the inflammatory reaction in carotid arteries after balloon injury, and sophocarpine could exhibit a protective role.
Discussion
Cigarette smoke exposure is one of the major causes of atherosclerosis-related diseases, which can exacerbate vascular inflammation and lumen stenosis (27). Previous research has shown that the rate of restenosis is increased following angioplasty among smokers compared to non-smokers. There are more than 5,000 chemical components found in cigarette smoke, of which at least 150 are potentially hazardous to the health of smokers (28,29). Hence, smoking-induced vascular damage can be resulted from the combined effects of different toxic substances in cigarette smoke, instead of nicotine alone. This study investigated the protective effects of sophocarpine on smoking-induced vascular damage and restenosis prevention after angioplasty. The experiments consisted of the following four characteristics: (I) the rats were placed under cigarette smoke to simulate the real smoking status, and the effects of smoking on vascular injury were observed; (II) the carotid arteries of rats with cigarette smoke exposure were subjected to balloon injury to imitate the situation of interventional angioplasty; (III) this study included the observation of smoking-induced morphologic changes, such as intimal thickening and lumen stenosis, during vascular injury within a short period of time, although it takes several years or even decades for cigarette smoke to cause such variations; and (IV) the morphologic variations were observed in rat carotid artery balloon injury model and the molecular mechanisms were studied in the contralateral carotid arteries in order to rule out the influence of balloon injury.
Atherosclerosis, mainly caused by smoking, has been characterized as inflammatory injury, oxidative injury, smooth muscle cell proliferation and migration, intimal thickening, and ultimately coronary lumen stenosis (30). In this study, we observed that cigarette smoking could directly cause the intimal thickening of the carotid arteries after balloon injury, and sophocarpine could ameliorate such intimal hyperplasia. In addition, our results revealed that cigarette smoking could induce vascular injury, lumen stenosis, and even restenosis following angioplasty. These morphologic variations were consistent with those of previous studies and clinical observations (8). The findings also implied that sophocarpine might confer protective effects on vascular damage induced by cigarette smoke and restenosis following angioplasty.
Considering that inflammation is involved during atherosclerosis, we elucidated the mechanisms underlying vascular damage induced by cigarette smoke and the beneficial effects of sophocarpine with regards to its anti-inflammatory activities. First, we observed that cigarette smoke significantly induced the levels of p-MKK3/6 and p-P38 in carotid arteries, and sophocarpine could inhibit such high levels of phosphorylated MKK3/6 and P38. It has been reported that matrine can suppress ox-LDL-induced inflammatory response in macrophages by inhibiting MKKs/p38 MAPK signaling pathway (31). Numerous studies have shown that p38 is a key mediator for the inflammation of various human endothelial cells (32,33). Moreover, the expression levels of TNF-α and IL-1β have been detected, and the results revealed that sophocarpine could suppress cigarette smoke-induced overexpression of TNF-α and IL-1β. It is worth noting that TNF-α and IL-1β are both participated in vascular inflammation (34). Previous studies have demonstrated that the mRNA levels of TNF-α and IL-1β can be down-regulated by inhibiting p38 MAPK signaling pathway (35,36). All these observations were consistent with our results, and thus, we can conclude that sophocarpine ameliorates vascular inflammation by activating MKKs/p38 MAPK signaling pathway.
In summary, our findings reveal that (I) cigarette smoking aggravates the stenosis of lumen in carotid arteries after balloon injury; (II) the mechanisms underlying smoking-induced vascular injury are related with inflammatory reaction; and (III) sophocarpine can ameliorate cigarette smoke-induced restenosis after angioplasty by down-regulating the expression levels of proinflammatory cytokines. Altogether, the findings of this study indicate a beneficial role of sophocarpine in vascular damage induced by cigarette smoke and supports the notion that sophocarpine exhibits favorable therapeutic potential for preventing restenosis in patients who continued to smoke after angioplasty.
Acknowledgments
Funding: This work was supported by the Fundamental Research Funds for the Central Universities (3332019028).
Footnote
Reporting Checklist: The authors have completed the 15-ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-19-568
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-19-568
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-19-568). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complied with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and the Animal Management Rule of the Chinese Ministry of Health, and was approved by the Animal Care Committee of Peking Union Medical College (JS-863).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Basu S, Stuckler D, Bitton A, et al. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. BMJ 2011;343:d5506. [Crossref] [PubMed]
- Roh S. Scientific Evidence for the Addictiveness of Tobacco and Smoking Cessation in Tobacco Litigation. J Prev Med Public Health 2018;51:1-5. [Crossref] [PubMed]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 2007;17:259-73. [Crossref] [PubMed]
- Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509-15. [Crossref] [PubMed]
- Drummond CA, Brewster PS, He W, et al. Cigarette smoking and cardio-renal events in patients with atherosclerotic renal artery stenosis. PLoS One 2017;12:e0173562. [Crossref] [PubMed]
- Gonçalves RB, Coletta RD, Silverio KG, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res 2011;60:409-24. [Crossref] [PubMed]
- Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res 2012;91:142-9. [Crossref] [PubMed]
- Yang GH, Li YC, Wang ZQ, et al. Protective effect of melatonin on cigarette smoke-induced restenosis in rat carotid arteries after balloon injury. J Pineal Res 2014;57:451-8. [Crossref] [PubMed]
- van Dijk WD, Akkermans R, Heijdra Y, et al. The acute effect of cigarette smoking on the high-sensitivity CRP and fibrinogen biomarkers in chronic obstructive pulmonary disease patients. Biomark Med 2013;7:211-9. [Crossref] [PubMed]
- Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 2010;34:J258-65. [Crossref] [PubMed]
- Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 2007;1773:1358-75. [Crossref] [PubMed]
- Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res 2007;86:800-11. [Crossref] [PubMed]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res 2005;15:11-8. [Crossref] [PubMed]
- Yang Y, Kim SC. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm 2014;2014:352371. [Crossref] [PubMed]
- Corre I, Paris F, Huot J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget 2017;8:55684-714. [Crossref] [PubMed]
- Reustle A, Torzewski M. Role of p38 MAPK in Atherosclerosis and Aortic Valve Sclerosis. Int J Mol Sci 2018;19:3761. [Crossref] [PubMed]
- Cai XH, Guo H, Xie B. Structural Modifications of Matrine-Type Alkaloids. Mini Rev Med Chem 2018;18:730-44. [Crossref] [PubMed]
- Pan QM, Li YH, Hua J, et al. Antiviral Matrine-Type Alkaloids from the Rhizomes of Sophora tonkinensis. J Nat Prod 2015;78:1683-8. [Crossref] [PubMed]
- Liu Z, Zhang Y, Tang Z, et al. Matrine attenuates cardiac fibrosis by affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur J Pharmacol 2017;804:21-30. [Crossref] [PubMed]
- Zheng P, Niu FL, Liu WZ, et al. Anti-inflammatory mechanism of oxymatrine in dextran sulfate sodium-induced colitis of rats. World J Gastroenterol 2005;11:4912-5. [Crossref] [PubMed]
- Li J, Li L, Chu H, et al. Oral sophocarpine protects rat heart against pressure overload-induced cardiac fibrosis. Pharm Biol 2014;52:1045-51. [Crossref] [PubMed]
- Zhang Y, Wang S, Li Y, et al. Sophocarpine and matrine inhibit the production of TNF-alpha and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int Immunopharmacol 2008;8:1767-72. [Crossref] [PubMed]
- Li C, Gao Y, Tian J, et al. Sophocarpine administration preserves myocardial function from ischemia-reperfusion in rats via NF-kappaB inactivation. J Ethnopharmacol 2011;135:620-5. [Crossref] [PubMed]
- Hautamaki RD, Kobayashi DK, Senior RM, et al. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002-4. [Crossref] [PubMed]
- Liu Z, Lv Y, Zhang Y, et al. Matrine-Type Alkaloids Inhibit Advanced Glycation End Products Induced Reactive Oxygen Species-Mediated Apoptosis of Aortic Endothelial Cells In Vivo and In Vitro by Targeting MKK3 and p38MAPK Signaling. J Am Heart Assoc 2017;6:e007441. [Crossref] [PubMed]
- Tulis DA, Durante W, Liu X, et al. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 2001;104:2710-5. [Crossref] [PubMed]
- Siasos G, Tsigkou V, Kokkou E, et al. Smoking and atherosclerosis: mechanisms of disease and new therapeutic approaches. Curr Med Chem 2014;21:3936-48. [Crossref] [PubMed]
- Thielen A, Klus H, Muller L. Tobacco smoke: unraveling a controversial subject. Exp Toxicol Pathol 2008;60:141-56. [Crossref] [PubMed]
- Braun KF, Ehnert S, Freude T, et al. Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes HO-1 and SOD-1. ScientificWorldJournal 2011;11:2348-57. [Crossref] [PubMed]
- McGill HC. Smoking and the pathogenesis of atherosclerosis. Adv Exp Med Biol 1990;273:9-16. [Crossref] [PubMed]
- Zhou J, Ma W, Wang X, et al. Matrine Suppresses Reactive Oxygen Species (ROS)-Mediated MKKs/p38-Induced Inflammation in Oxidized Low-Density Lipoprotein (ox-LDL)-Stimulated Macrophages. Med Sci Monit 2019;25:4130-6. [Crossref] [PubMed]
- Gong L, Lei Y, Liu Y, et al. Vaccarin prevents ox-LDL-induced HUVEC EndMT, inflammation and apoptosis by suppressing ROS/p38 MAPK signaling. Am J Transl Res 2019;11:2140-54. [PubMed]
- Koch SR, Choi H, Mace EH, et al. Toll-like receptor 3-mediated inflammation by p38 is enhanced by endothelial nitric oxide synthase knockdown. Cell Commun Signal 2019;17:33. [Crossref] [PubMed]
- Stanisic M, Majewski W, Zurawski J, et al. IL-6, TNF-alpha, IL-1-beta arterial wall expression is independent of serum concentration in patients sustaining primary or secondary open vascular reconstructions. Int Angiol 2010;29:496-506. [PubMed]
- Kim DO, Byun JE, Seong HA, et al. Thioredoxin-interacting protein-derived peptide (TN13) inhibits LPS-induced inflammation by inhibiting p38 MAPK signaling. Biochem Biophys Res Commun 2018;507:489-95. [Crossref] [PubMed]
- Dong H, Cui B, Hao X. MicroRNA22 alleviates inflammation in ischemic stroke via p38 MAPK pathways. Mol Med Rep 2019;20:735-44. [PubMed]


