
Long-term compound danshen dripping pills therapy reduces the no-reflow phenomenon in nondiabetes mellitus patients after primary percutaneous coronary intervention for acute myocardial infarction
Introduction
An increasing number of patients have received primary percutaneous coronary intervention (PPCI) in China in recent years, and specific problems, especially no-reflow phenomenon, associated with PPCI have drawn greater attention. In the Western world, the incidence of the no-reflow phenomenon ranges from 5% to 50% (1), while in Asia it ranges from 5% to 37% (2,3). Regardless of this variability, the no-reflow phenomenon after PPCI is not a rare event. In recent years, research on how to predict and treat the no-reflow phenomenon has been conducted by many investigators (2-8). Previous studies have demonstrated that compound danshen dripping pills (CDDP) is effective for patients with angina pectoris and its active constituents have bioactivities against myocardial ischemia, inflammation, and angiotensin-converting enzyme which could protect human umbilical vein endothelial cells from injury induced by lipopolysaccharide. Here, we report our retrospective study that was aimed at assessing the effect of CDDP, a popular Chinese traditional medicine, on reducing the no-reflow phenomenon. We further identified a few admission parameters as predictors of the no-reflow phenomenon in Chinese patients undergoing PPCI. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1516).
Methods
Study population
In total, 425 consecutive patients were selected from 3,361 patients undergoing PPCI for treatment of coronary heart disease (between January 2009 and March 2012) according to the following inclusion criteria: (I) suffering from acute myocardial infarction (AMI); (II) no diabetes mellitus (DM) on admission; (III) no contraindication for CDDP; (IV) received drug-eluting stents implantation. The diagnosis of AMI was confirmed by angiography. Pre-infarction angina refers to cardiac symptoms lasting less than 30 min that occur within 48 h before onset of infarction. Among these 425 patients, 26 patients were excluded because their treatments with CDDP all occurred earlier than 1 year before PPCI. All procedures performed in this study were in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital (lot. 20121202).
PPCI and the no-reflow phenomenon
Pharmacological treatment before PPCI was done according to the following Chinese guidelines: 300 mg aspirin (chewed, Bayer) and 300 mg oral clopidogrel (Sanofi Aventis) immediately after admission, and intravenous unfractionated heparin at a dose of 70 U/kg of body weight. PPCI procedures were performed by experienced doctors using femoral or radial approach, and drug-eluting stents were implanted in all patients. All patients were treated with maintenance doses of aspirin (100 mg once daily indefinitely) and clopidogrel (75 mg once daily for 1 year). Other medications (including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, and β-receptor blockers) were administered according to guidelines. Angiograms were analyzed using a validated quantitative coronary angiographic system (MEDIS, CMS 4.0, Leiden, The Netherlands). Myocardial blush grade immediately after PPCI was evaluated by 2 experienced investigators, who were otherwise blinded to all clinical data. The perfusion status of the infarction-related artery (IRA) was assessed in accordance with the myocardial blush grade. No-reflow phenomenon was defined as a thrombolysis in myocardial infarction (TIMI) flow grade <3 or 3 with an MBG of 0–1.
Statistics
All statistical data were analyzed using the SPSS 16.0 (SPSS-PC Inc., Chicago, IL, USA) software package. Categorical variables are expressed as number and percentage of patients while continuous variables are expressed as mean ± standard deviation (all continuous variables with normal distribution were assessed by Kolmogorov-Smirnov test) and compared using Student’s t-test or analysis of variance (ANOVA) as appropriate. Categorical variables were compared using Chi-square statistics or Fisher’s exact test. Multivariate stepwise logistic regression analysis was used to identify independent predictors for the no-reflow phenomenon. A value of P<0.05 (2-sided) was considered statistically significant.
Results
Baseline characteristics
A total of 399 consecutive patients undergoing PPCI for AMI were identified. Among the 399 patients (mean age, 59.5±11.7 years old), 293 patients (73.4%) were male. In this study, we excluded patients with DM because DM is a contraindication for CDDP. Firstly, patients were divided into 2 groups: the CDDP group, defined as long-term treatment with CDDP; and the NCDDP, defined as not treated with CDDP within a year before PPCI. Clinical characteristics of both groups are summarized in Table 1. As can be seen, there is a significant difference of no-reflow incidence between these 2 groups (9/68 vs. 87/331, 13.2% vs. 26.3%, P=0.0219). Also, we found that the serum levels of high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and brain natriuretic peptide (BNP) in the CDDP group were significantly lower than those in the NCDDP group (P=0.0270, 0.0155, and 0.0032, respectively).
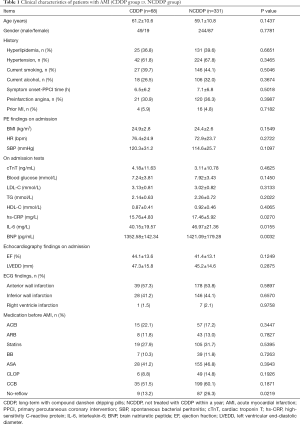
Full table
No-reflow phenomenon and long-term CDDP treatment
The no-reflow phenomenon occurred in 96 patients, and 24.1% of 399 non-DM patients undergoing PPCI. Patients were then divided into 2 groups: the no-reflow group (n=96) and the reflow group (n=303). Clinical characteristics of these 2 groups are summarized in Table 2. In a comparison of the no-reflow group and the reflow group, the following items were found to be statistically different: number of prior myocardial infarction (MI), systolic blood pressure (SBP), cardiac troponin T (cTnT), hs-CRP, BNP, and IL-6 on admission, ejection fraction (EF), left ventricular end-diastolic diameter (LVEDD), number of anterior wall infarctions, and number of patients with long-term CDDP treatment (P all <0.05, Table 2).
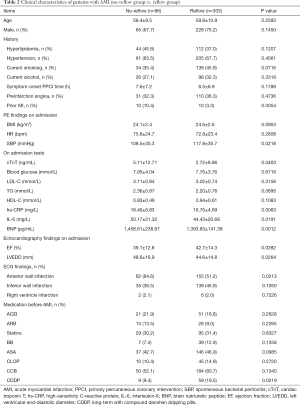
Full table
Predictors of no-reflow phenomenon
As mentioned above, number of prior MI, SBP, cTnT, hs-CRP, BNP, and IL-6 on admission, EF, LVEDD, number of anterior wall infarctions, and number of patients with long-term CDDP treatment were significantly different between the no-reflow group and the reflow group, according to univariable analysis. Further multivariate stepwise logistic regression analysis indicated that these factors were all independent predictors of the no-reflow phenomenon in non-DM patients receiving PPCI (Table 3). With an odds ratio (OR) of 0.44 (95% CI: 0.89–0.17, P=0.0176), long-term treatment with CDDP appeared to be a protective factor for the no-reflow phenomenon.
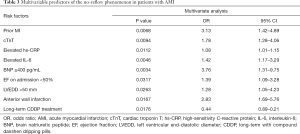
Full table
Discussion
In this study, we firstly showed that patients with long-term CDDP treatment had a lower incidence of the no-reflow phenomenon than non-CDDP patients (13.2% vs. 26.3%, P=0.0219). The results also revealed that patients with long-term CDDP treatment had lower serum levels of hs-CRP, IL-6, and BNP. Subsequent multivariate stepwise logistic regression analysis demonstrated that long-term CDDP treatment was an independent predictor for the no-reflow phenomenon (OR =0.44, 95% CI: 0.89–0.21). Thus, for the first time, our study demonstrated that long-term treatment with CDDP, a popular Chinese traditional medicine, could reduce the no-reflow phenomenon in non-DM patients who underwent PPCI because of AMI.
The no-reflow phenomenon has been demonstrated to be associated with a poor prognosis after primary coronary intervention in patients with AMI. No-reflow predicts early post-infarction complications, reduces myocardial salvage, increases infarct size, worsens left ejection fraction, and increases mortality. The underlying mechanism of no-reflow includes: (I) distal atherothrombotic embolization; (II) ischemic injury; (III) reperfusion injury; and (IV) individual predisposition of coronary microcirculation to injury (1). CDDP have been used in China for a long time. Some randomized controlled trials have shown that CDDP treatment is more effective than isosorbide dinitrate in treating angina pectoris (9). Abundant research has demonstrated that CDDP has multiple effects and therapeutic targets in the cardiovascular system, meaning it has a potential effect on the no-reflow phenomenon. Danshen (which is the main ingredient of CDDP) and its active tanshinones and isotanshinones compounds have bioactivities against myocardial ischemia, inflammation, and angiotensin-converting enzyme (10). An in vitro study revealed that CDDP could protect human umbilical vein endothelial cells from injury induced by lipopolysaccharide (11). Pan et al. found that salvianolic acid A, the water-soluble component from the root of danshen prevents ischemia/reperfusion-induced myocardial damage by reducing necrosis and apoptosis in isolated rat hearts and cardiomyocytes. This effect was partially associated with the Akt signaling pathway (12). In another study, Huang et al. demonstrated that salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase (13). Another in vivo study found that after treatment with CDDP (20 pills, bid or 10 pills tid) for 3 months, endothelium-dependent vasodilation of the brachial artery improved significantly in patients with a high risk of coronary heart disease (14,15). CDDP also could increase the serum level of nitric oxide and 6-Keto-PGF-1a while decreasing the serum level of endothelin (ET) and thromboxane B2 (TXB2), which indicates improvement of endothelium function (15). Our results, together with these previous results, indicate preventive effect of CDDP for no-reflow phenomenon.
Our data also revealed some independent predictors of no-reflow phenomenon. Among these factors, prior MI, SBP, hs-CRP, BNP on admission, EF, LVEDD, and anterior wall infarction have been reported in previous studies (2-8). Our results were consistent with these studies. Meanwhile, in our study, cTnT and IL-6 on admission were reported for the first time to be predictors of no-reflow phenomenon. Previous studies have demonstrated that IL-6 could downregulate tissue factors pathway inhibitors in vascular endothelial cells (16). Subgroup analysis of the IABP-SHOCL-trial showed that IL-6, together with other inflammatory factors including IL-7, -8, and -10, could be a valuable predictor for mortality of infarct-related cardiogenic shock patients (17). IL6R signaling seems to have a causal role in the development of coronary heart disease and IL6R blockade could provide a novel therapeutic approach to prevention of coronary heart disease (18). Actually, IL-6 is one of the most important stimuli of CRP release. IL-6 has also been shown to significantly correlate with increased left atrial size, an important risk factor for developing atrial fibrillation (19). All these findings suggest that IL-6 possibly plays an important role in the no-reflow phenomenon. As for cTnT, Giannitsis et al. found that troponin T on admission level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after PPCI for acute ST-segment elevation myocardial infarction (20). However, they did not precisely analyze the relationship of cTnT with the no-reflow phenomenon. Later, Nguyen et al. demonstrated that elevated cTnT on admission was associated with a higher corrected TIMI frame count (P=0.04) and with a trend toward worse myocardial blush grade at the end of the procedure (P=0.069), indicating a higher degree of microvascular obstruction, which is a possible mechanism of the no-reflow phenomenon (1,21).
According to the results from previous studies and our present research, a combination of some regular on-admission parameters including CRP, IL-6, cTnT, and BNP, with other physical examination and echocardiography findings before PPCI, may provide a more powerful tool to predict the no-reflow phenomenon in advance of PPCI. This hypothesis needs to be investigated and confirmed in a large population of this patient type in the future.
Some limitations in our study should also be addressed. The study population in our present research seemed to be relatively small and many patients were excluded because of DM which is the main coexisting disease of coronary heart disease. Meanwhile, since this was a retrospective study, it was possible that there were some variation in parameters from the blood test. A strictly designed prospective study is needed in the future to further evaluate the protective effect of CDDP on the no-reflow phenomenon.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1516
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1516
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1516). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital (lot. 20121202).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rezkalla SH, Stankowski RV, Hanna J, et al. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc Interv 2017;10:215-23. [Crossref] [PubMed]
- Zhao JL, Fan CM, Yang YJ, et al. Chronic Pretreatment of Metformin is Associated with the Reduction of the No-Reflow Phenomenon in Patients with Diabetes Mellitus After Primary Angioplasty for Acute Myocardial Infarction. Cardiovasc Ther 2013;31:60-4. [Crossref] [PubMed]
- Jeong YH, Kim WJ, Park DW, et al. Serum B-type natriuretic peptide on admission can predict the 'no-reflow' phenomenon after primary drug-eluting stent implantation for ST-segment elevation myocardial infarction. Int J Cardiol 2010;141:175-81. [Crossref] [PubMed]
- Kobayashi A, Misumida N. Percutaneous coronary intervention in left main disease: 10-year follow-up. Ann Transl Med 2019;7:85. [Crossref] [PubMed]
- Magro M, Nauta ST, Simsek C, et al. Usefulness of the SYNTAX score to predict "no reflow" in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol 2012;109:601-6. [Crossref] [PubMed]
- Fajar JK, Heriansyah T, Rohman MS. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: A meta-analysis. Indian Heart J 2018;70 Suppl 3:S406-418. [Crossref] [PubMed]
- Sgueglia GA, Niccoli G, Spaziani C, et al. Baseline von Willebrand factor plasma levels and no-reflow phenomenon after primary percutaneous coronary intervention for ST segment elevation myocardial infarction. Int J Cardiol 2010;145:230-2. [Crossref] [PubMed]
- Erkol A, Oduncu V, Pala S, et al. Plasma osteoprotegerin level on admission is associated with no-reflow phenomenon after primary angioplasty and subsequent left ventricular remodeling in patients with acute ST-segment elevation myocardial infarction. Atherosclerosis 2012;221:254-9. [Crossref] [PubMed]
- Jia Y, Huang FY, Zhang SK, et al. Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. Int J Cardiol 2012;157:330-40. [Crossref] [PubMed]
- Li ZM, Xu SW, Liu PQ. Salvia miltiorrhizaBurge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol Sin 2018;39:802-24. [Crossref] [PubMed]
- Wang DX, Wang XM, Xu JL. The protective effect of composite salviae dropping pills on human umbilical vein endothelial cells injured by lipopolysaccharide. Chin J Pathophysiol 2006;22:933-7.
- Pan H, Li D, Fang F, et al. Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J Cardiovasc Pharmacol 2011;58:535-42. [Crossref] [PubMed]
- Huang ZS, Zeng CL, Zhu LJ, et al. Salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase. J Thromb Haemost 2010;8:1383-93. [Crossref] [PubMed]
- Ge YX, Duan YY, Ruan LT, et al. Protective effect of the compound danshen diwan on endothelium function of blood vessel. Chin J Clin Rehab 2004;8:1096-7.
- Chen HQ, Xiong XQ, Duan ZH, et al. Effect of compound danshen dropping pills on endothelium function in patients with coronary heart disease. J Sun Yat-Sen University 2009;30:221-8. (Medical Sciences).
- Tang XL, Jiang ZY, Cai SY, et al. IL-6 inhibits tissue factor pathway inhibitor expression in human umbilical vein endothelial cells. Acad J Sec Mil Med Univ 2005;26:147-50.
- Mora-Ruíz MD, Blanco-Favela F, Chávez Rueda AK, et al. Role of interleukin-17 in acute myocardial infarction. Mol Immunol 2019;107:71-8. [Crossref] [PubMed]
- The Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379:1214-24. [Crossref]
- Marcus GM, Whooley MA, Glidden DV, et al. Interleukin 6 and Atrial Fibrillation in Patients with Coronary Artery Disease: Data from the Heart and Soul Study. Am Heart J 2008;155:303-9. [Crossref] [PubMed]
- Giannitsis E, Müller-Bardorff M, Lehrke S, et al. Admission troponin T level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after primary percutaneous intervention for acute ST-segment elevation myocardial infarction. Circulation 2001;104:630-5. [Crossref] [PubMed]
- Nguyen TL, Phan JA, Hee L, et al. High-sensitivity troponin T predicts infarct scar characteristics and adverse left ventricular function by cardiac magnetic resonance imaging early after reperfused acute myocardial infarction. Am Heart J 2015;170:715-25.e2. [Crossref] [PubMed]


