
3D-STI evaluation of the effect of dexrazoxane on the mechanical properties of right ventricular myocardium in breast cancer patients treated with pirarubicin
Introduction
Breast cancer is one of the most common types of malignant tumor worldwide. With an annually increasing prevalence rate, it poses a serious threat to human health. In 2018, breast cancer was second only to lung cancer by incidence and the mortality rate is first in women (1,2). Postoperative adjuvant chemotherapy is an effective treatment for breast cancer. Currently, anthracyclines are included in the first-choice postoperative chemotherapy plan for breast cancer patients (3). Although anthracyclines can significantly improve the survival rate of breast cancer patients and reduce recurrence and metastasis, it can induce dose-related (4) myocardial toxicity, which causes irreversible heart damage (5-7).
At an early stage, monitoring cardiac function is based entirely on relatively insensitive indicators such as symptoms and left ventricular ejection fraction (LVEF), which may lead to the opportunity for early detection and timely treatment being missed (4,8,9). Therefore, there has been a considerable amount of research centered on anthracycline-induced changes in left ventricular overall function during chemotherapy after breast cancer surgery (10-14). In contrast, studies on the changes in right ventricular overall and local function are limited (15). Nevertheless, the structure and overall and local functions of the right ventricle are highly important to the incidence rate and mortality of heart and lung diseases.
Three-dimensional speckle-tracking imaging (3D-STI) is a new technology that combines two-dimensional speckle-tracking imaging (2D-STI) with real-time three-dimensional echocardiography (RT-3DE) and possesses the advantages of these two techniques (16). It can track speckles in three dimensions (simultaneously eliminating noise and improving accuracy) through acquisition and can reflect the function and movement of the heart muscle with higher precision, facilitating more accurate evaluation of the overall and local structure, movement, and function of the heart (17,18). Dexrazoxane is an antitumor adjuvant that can be used to alleviate or reduce the cardiotoxicity induced by chemotherapy with anthracycline antibiotics (such as doxorubicin). For improved clinical judgment of patients’ conditions, treatment selection, and prognostic evaluation, the accurate and quantitative evaluation of the right ventricular structure and function is especially important (19,20). In this study, 3D-STI was used to evaluate the effect of dexrazoxane based on the mechanical properties of the right ventricular myocardium in breast cancer patients who received pirarubicin chemotherapy after radical mastectomy, and its clinical value was explored.
We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1074).
Methods
Research subjects
Between January 2017 and December 2018, 64 patients with invasive breast cancer who were hospitalized for chemotherapy (with regular CTF chemotherapy) in the Thyromammary Surgery Department of our hospital were selected for this prospective study. The patients had an average age of 33.2±8.3 years (22–43 years) old. Using a random number table, the patients were randomly divided into two groups, the experimental group (with dexrazoxane added) and the control group (without dexrazoxane), with 32 patients in each group.
The inclusion criteria, all of which had to be met, were as follows: (I) normal indexes of liver and kidney function, electrocardiograph (ECG) and chest radiograph; (II) conventional echocardiography showed no significant abnormalities (including cardiac function and atrioventricular size); (III) no multiple distant metastases; (IV) had not previously received pirarubicin; and (V) had not previously received radiotherapy (15).
Patients who met any one of the following criteria were excluded: (I) organic heart disease (including congenital heart disease and acquired heart disease); (II) hypertension; (III) arrhythmia; (IV) diabetes, hyperthyroidism, hypothyroidism and other endocrine diseases; (V) hyperlipidemia; (VI) pulmonary hypertension; (VII) other malignant tumors; or (VIII) pregnant or lactating.
All procedures performed in this study were in accordance with the Declaration of Helsinki and this study was approved by the medical ethics committee of our hospital. All selected patients were informed and signed informed consent was obtained from each patient.
Research content
Instruments and methods
Echocardiography was performed with Color Doppler Ultrasonic Diagnosis Apparatus (Toshiba Artida SSH-880CV) equipped with three-dimensional analysis software. The subjects were instructed to lie on their left side and to breathe calmly while 12-lead routine ECG was recorded in a quiet state. Conventional echocardiography (PST-30SBT probe, frequency 2.5–5.0 MHz) combined with three-dimensional data acquisition (PST-25SX probe, frequency 1–3 MHz, frame volume 20–25 frames per second) was performed. The data were measured in triplicate, collected and averaged.
Acquisition of conventional parameters of the right ventricle
According to the consensus of the American Society of echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) on the updated Guidelines for the Diagnosis of Adult Echocardiography published in 2015, the conventional echocardiography parameters of the right ventricle were measured as follows: right ventricular fractional area change (RVFAC); tricuspid annular plane systolic excursion (TAPSE) measured in M-type; and Doppler tricuspid annulus systolic blood flow velocity of tissues (S’) measured by Tissue Doppler Imagine (TDI).
Acquisition of 3D-STI parameters of the right ventricle
The “pre-4D” key was pressed to display the real-time and double images of the right ventricular apical two-chamber view and the right ventricular apical four-chamber view. When the right ventricular epicardium and endocardium were clearly and completely visible, the “Full-4D” key was pressed and the ECG was simultaneously triggered to start the three-dimensional data acquisition program, which mainly monitors the RVGLS, right ventricle global circular strain (RVGCS), right ventricle global radial strain (RVGRS), RVGAS (Figure 1).
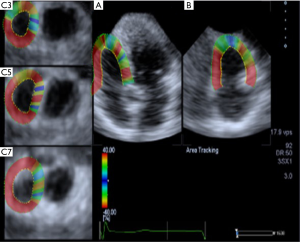
Serological testing
On the day before the chemotherapy commenced and on the morning after the completion of the chemotherapy, 2 mL of peripheral venous blood was collected from the patients (who were fasting). The serum was separated from each sample within 1 h. The levels of hs-cTnI and N-terminal B-type pro-natriuretic peptide (NT-proBNP) were measured using Vidas-Blue automatic fluorescence immunoassay analyzer.
Statistical analysis
All data were statistically processed with SPSS 24.0 statistical software. The measurement data were expressed as mean ± standard deviation. The differences between the two groups were tested by independent-samples t-test, and the differences between the groups before and after treatment were tested by paired sample t-test. The level of significance was α=0.05.
Results
Comparison of patients before and after chemotherapy in the experimental group
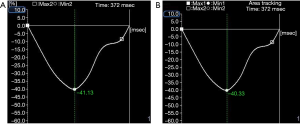
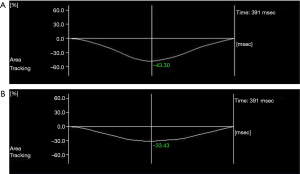
- In the experimental group, RVGLS (−26.10%±2.33% vs. −28.60%±2.57%) and RVGAS (−35.78%±5.60% vs. −38.66%±5.73%) were significantly lower after chemotherapy than before chemotherapy (P<0.05). However, there were no significant differences in RVGCS (−17.79%±2.26% vs. −17.85%±2.25%), RVGRS (74.72%±13.46% vs. 74.83%±13.49%), hs cTnI (0.153±0.061 vs. 0.151±0.062 ng/mL), or NT-proBNP (68.10±15.21 vs. 67.66±15.10 pg/mL) (P>0.05, Table 1, Figure 2).
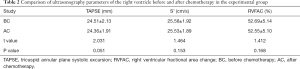
- In the experimental group, there were no significant differences in tricuspid annulus systolic displacement (24.51±2.13 vs. 24.36±1.91 mm), Doppler tricuspid annulus systolic blood flow velocity of tissues (25.58±1.92 vs. 25.53±1.89 cm/s), or change fraction of right ventricular area (52.69%±5.14% vs. 52.55%±5.10%) before and after chemotherapy (P>0.05, Table 2).

Full table
Full table
Comparison before and after chemotherapy in the control group
- In the control group, RVGLS (−24.59%±2.36% vs. −28.22%±2.15%) and RVGAS (−32.77%±6.23% vs. −36.40%±6.38%) after chemotherapy were significantly lower than before chemotherapy (P<0.05). However, there were no significant differences in RVGCS (−18.17%±2.20% vs. −18.22%±2.21%), RVGRS (74.64%±13.44% vs. 74.73%±13.54%), hs cTnI (0.171±0.073 vs. 0.166±0.065 ng/mL), and NT-proBNP (68.79±15.68 vs. 67.84±15.11 pg/mL) (P>0.05, Table 3, Figure 3).
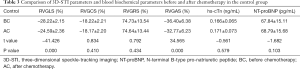
- In the control group, there was were no significant differences in tricuspid annulus systolic displacement (TAPSE 24.65±2.11 vs. 24.49±1.85 mm), Doppler tricuspid annulus systolic blood flow velocity of tissues (S’ 25.88±1.98 vs. 25.78±1.87 cm/s), or change fraction of the right ventricular area (RVFAC 53.02%±5.49% vs. 52.91%±5.04%) before and after chemotherapy (P>0.05, Table 4).
Full table

Full table
Comparison between the data before and after chemotherapy in the experimental group and the control group
- Before chemotherapy, there were no significant differences between the experimental group and the control group in RVGLS (−28.60%±2.57% vs. −28.22%±2.15%), RVGCS (−17.85%±2.25% vs. −18.22%±2.21%), RVGRS (74.83%±13.49% vs. 74.73%±13.54%), RVGAS (−38.66%±5.73% vs. −36.40%±6.38%), hs-cTnI (0.151±0.062 vs. 0.166±0.065 ng/mL), NT-proBNP (67.66±15.10 vs. 67.84±15.11 pg/mL), TAPSE (24.51±2.13 vs. 24.65±2.11 mm), S’ (25.58±1.92 vs. 25.88±1.98 cm/s), or RVFAC (52.69%±5.14% vs. 53.02%±5.49%) (P>0.05).
- After chemotherapy, RVGLS (−26.10%±2.33% vs. −24.59%±2.36%) and RVGAS (−35.78%±5.60% vs. −32.77%±6.23%) were significantly different between the experimental group and the control group (P<0.05). However, there were no significant differences between the experimental group and the control group in RVGCS (−17.79%±2.26% vs. −18.17%±2.20%), RVGRS (74.72%±13.46% vs. 74.64%±13.44%), hs cTnI (0.153±0.061 vs. 0.171±0.073 ng/mL), NT-proBNP (68.10±15.21 vs. 68.79±15.68 pg/mL), TAPSE (24.36±1.91 vs. 24.49±1.85 mm), S’ (25.53±1.89 vs. 25.78±1.87cm/s), or RVFAC (52.55%±5.10% vs. 52.91%±5.04%) (P>0.05, Table 5).
Full table
Discussion
The structure of the right ventricular myocardium comprises three layers: the superficial layer, the middle layer, and the deep layer. In the superficial layer and deep layer of the cardiac fiber stent, the right ventricular myocardium spirals in the longitudinal direction. Therefore, during the systole of the heart, it moves toward the direction of the ventricular outflow tract (contraction of the superficial layer and deep layer of the right ventricular myocardium), causing the ventricle to move toward the bottom of the heart and shortening the cardiac cavity. With the synergistic effect of the ventricular septum, the blood is driven from the right ventricle into the pulmonary artery. The cardiac cavity is shortened by the contraction of the annular middle layer of the right ventricular myocardium. The longitudinal myocardium (the superficial and deep layers) of the right ventricle serves a crucial role in the cardiac ejection function (21,22).
Basic research has confirmed that the subendocardial longitudinal myocardial fibers of the heart can be affected by many factors, including such as ischemia and poisoning. It is possible because that anthracycline drugs first reach the subendocardial myocardium, the drug concentration in the microvessels of the endocardial side is high and the impact of blood flow on the subendocardial myocardium is relatively strong. Furthermore, because of the subendocardial myocardium’s mainly longitudinal direction, the ability of longitudinal deformation of the subendocardial myocardium may have already been damaged. In addition, it may also be aggravated by drug accumulation (23,24). In our study, RVGCS and RVGRS were not significantly reduced by pirarubicin chemotherapy (P>0.05), probably because the annular muscle in the middle layer of the myocardium is not obviously affected and the wall of the right ventricle is thin, which is consistent with the findings reported by Xu et al. (25) and Hu et al. (26), and differs from those reported by Zhang et al. (15). RVGAS is has higher sensitivity and can more accurately reflect the myocardial damage, because it comprehensively takes the longitudinal and circular motion of myocardium into account (27).
In this study, the mechanical properties of the right ventricular myocardium and the changes after the use of dexrazoxane combined with anthracycline drugs for chemotherapy in breast cancer patients following radical mastectomy were evaluated by three-dimensional speckle-tracking ultrasonography, with the aim of guiding the choice of clinical treatment.
It is currently believed that the main mechanism of anthracycline toxicity is topoisomerase 2β inhibition, which activates the cell death pathway and inhibits the biological origin of mitochondria. The combination of dexrazoxane and anthracycline drugs can interfere with topoisomerase 2β binding and reduce the toxicity and the risk of subsequent heart failure in high-risk patients (28).
At present, dexrazoxane is the only protective agent against cardiotoxicity caused by Food and Drug Administration (FDA)-approved anthracycline drugs (it has been listed in the Guidelines for Clinical Operation of Cancer Chemotherapy and Radiotherapy Protective Agents in the United States (29). Traditionally, it has also been recognized as a precursor drug of ADR-925, a metabolite of iron chelation (30).
As a cytotoxic drug, by blocking the catalytic activity of DNA topoisomerase-II and the average distribution of DNA monomers during mitosis (31,32), dexrazoxane exerts an antitumor mechanism. As an ethylenediaminetetraacetic acid analogue, dexrazoxane is hydrolyzed into an open-loop product of ICRF-198 in cardiomyocytes. The chelation of ICRF-198 and some intermediates with ions can inhibit Fe3+-pirarubicin chelate, reduce the induced production of free radicals, and ultimately inhibit the cardiotoxicity of pirarubicin (33,34). It therefore exerts a protective effect on the myocardium (35-39), reduces myocardial cell death, alleviates the degree of inflammatory response, and reduces tension in the blood vessels (40). Roti Roti et al. (41) showed that dexrazoxane inhibited the catalytic activity of topoisomerase II, leading to the decrease of the toxic effect of pirarubicin in mouse ovarian cells. Experimental (42) and clinical (34,43,44) studies have shown that the dexrazoxane in appropriate dose proportions with pirarubicin can effectively reduce the cardiotoxicity caused by the latter such as reduces the incidence of heart events and congestive heart failure (45). Moreover, dexrazoxane does not undermine the effect of pirarubicin (46,47), does not significantly increase the toxicity of drugs, and does not have any adverse effect on the survival rate (44), or increase the incidence of secondary malignant tumors (46,47). Dexrazoxane not only offers protection from the cardiotoxicity caused by epirubicin (48,49), but may also be used to treat pre-existing myocardial damage (50). Jirkovský et al. believed that early use of dexrazoxane could effectively prevent and reduce the cardiotoxicity caused by anthracyclines in breast cancer patients (51).
Conclusions
- Despite there being no significant change in the right ventricular systolic function of breast cancer patients who underwent chemotherapy with pirarubicin after radical mastectomy, 3D-STI showed that the lRVGLS and RVGAS had decreased, which suggested that the mechanical properties of the right ventricular myocardium had been damaged.
- Before chemotherapy, there was no significant difference in the ultrasonography parameters and serological indexes between patients who received and those who did not receive dexrazoxane before chemotherapy. However, at the end of chemotherapy, the RVGLS and RVGAS in the group who did not receive dexrazoxane were significantly lower than those of the group who received dexrazoxane. This indicated that dexrazoxane could alleviate or remove the toxicity of pirarubicin in the right ventricular myocardium.
- It is expected that 3D-STI will offer a new method for the early and accurate evaluation of the mechanical properties and functional changes of the right ventricular myocardium in patients who receive pirarubicin chemotherapy after breast cancer surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1074
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1074
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1074). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki and this study was approved by the medical ethics committee of our hospital (No. LW20161210001). All selected patients were informed and signed informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deshmukh SK, Srivastava SK, Poosarla T, et al. Inflammation, immunosuppressive microenvironment and breast cancer: opportunities for cancer prevention and therapy. Ann Transl Med 2019;7:593. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Jain D, Aronow W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp Pract 1995;2019:6-15. [PubMed]
- Yu AF, Ky B. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart 2016;102:425-30. [Crossref] [PubMed]
- Angsutararux P, Luanpitpong S, Issaragrisil S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid Med Cell Longev 2015;2015:795602. [Crossref] [PubMed]
- Naito Y, Urasaki T. Precision medicine in breast cancer. Chin Clin Oncol 2018;7:29. [Crossref] [PubMed]
- Caron J, Nohria A. Cardiac Toxicity from Breast Cancer Treatment: Can We Avoid This. Curr Oncol Rep 2018;20:61. [Crossref] [PubMed]
- Cardinale D, Colombo A, Bacchiani G, et al. Response to Letters Regarding Article, “Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy Circulation 2016;133:e363. [Crossref] [PubMed]
- Laursen AH, Thune JJ, Hutchings M, et al. 123 I-MIBG imaging for detection of anthracycline-induced cardiomyopathy. Clin Physiol Funct Imaging 2018;38:176-85. [Crossref] [PubMed]
- Zhang S, Xu X, Zhang K, et al. Evaluation of heart function of breast cancer patients after chemotherapy with anthracyclines by 3D speckle tracking imaging. Chinese Journal of Medical Imaging 2014;(10):745-8+753.
- Jiang Y, Huang P, Yan Li, et al. Evaluation of left ventricular function in different chemotherapy cycles of breast cancer by 3D speckle tracking technique. Medical Innovation of China 2015;12:69-71.
- Clasen SC, Scherrer-Crosbie M. Applications of left ventricular strain measurements to patients undergoing chemotherapy. Curr Opin Cardiol 2018;33:493-7. [PubMed]
- Gripp EA, Oliveira GE, Feijó LA, et al. Global Longitudinal Strain Accuracy for Cardiotoxicity Prediction in a Cohort of Breast Cancer Patients During Anthracycline and/or Trastuzumab Treatment. Arq Bras Cardiol 2018;110:140-50. [PubMed]
- Kostakou PM, Kouris NT, Kostopoulos VS, et al. Cardio-oncology: a new and developing sector of research and therapy in the field of cardiology. Heart Fail Rev 2019;24:91-100. [Crossref] [PubMed]
- Zhang P, Lu G, Huang L, et al. Evaluation of ventricular function in breast cancer patients before and after chemotherapy with anthracyclines by 3D speckle tracking imaging. Chinese Journal of Ultrasonography 2017;26:17-20.
- Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 2011;12:167-205. [Crossref] [PubMed]
- Hayat D, Kloeckner M, Nahum J, et al. Comparison of real-time three-dimensional speckle tracking to magnetic resonance imaging in patients with coronary heart disease. Am J Cardiol 2012;109:180-6. [Crossref] [PubMed]
- Urbano-Moral JA, Patel AR, Maron MS, et al. Three-dimensional speckle-tracking echocardiography: methodological aspects and clinical potential. Echocardiography 2012;29:997-1010. [Crossref] [PubMed]
- Vitarelli A, Terzano C. Do we have two hearts? New insights in right ventricular function supported by myocardial imaging echocardiography. Heart Fail Rev 2010;15:39-61. [Crossref] [PubMed]
- Cameli M, Righini FM, Lisi M, et al. Right ventricular strain as a novel approach to analyze right ventricular performance in patients with heart failure. Heart Fail Rev 2014;19:603-10. [Crossref] [PubMed]
- Franco V. Right ventricular remodeling in pulmonary hypertension. Heart Fail Clin 2012;8:403-12. [Crossref] [PubMed]
- D'Andrea A, D'Alto M, Di MM, et al. Right atrial morphology and function in patients with systemic sclerosis compared to healthy controls: a two-dimensional strain study. Clin Rheumatol 2016;35:1733-42. [Crossref] [PubMed]
- Bi X, Deng Y, Zeng F, et al. Evaluation of left ventricular systolic function in breast cancer patients after epirubicin chemotherapy by two-dimensional strain echocardiography. Chinese Journal of Medical Imaging Technology 2009.25:1415-8
- Shi J, Pan C, Shu X, et al. Quantitative evaluation of left ventricular myocardial stratification strain in normal adults by two-dimensional speckle tracking imaging. Chinese Journal of Ultrasonography 2015;(2):378-81.
- Xu Y, Feng S, Chen J, et al. Evaluation of right ventricular function in uremic peritoneal dialysis patients by three-dimensional speckle tracking technique. Chinese Journal of Ultrasonography 2014;23:109-12.
- Hu L, Tian X, Zhou X, et al. Evaluation of right ventricular systolic function in patients with pulmonary hypertension by three-dimensional speckle tracking imaging. Chinese Journal of Ultrasound in Medicine 2015;31:974-7.
- Wen H, Liang Z, Zhao Y, et al. Feasibility of detecting early left ventricular systolic dysfunction using global area strain: a novel index derived from three-dimensional speckle-tracking echocardiography. Eur J Echocardiogr 2011;12:910-6. [Crossref] [PubMed]
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 2018;104:971-7. [Crossref] [PubMed]
- Wang B, Yuan X, Wang L, et al. Cardioprotection of dexrazoxane in adjuvant chemotherapy for elderly breast cancer patients. Journal of Modern Oncology 2016;24:2391-3.
- Jirkovský E, Jirkovská A, Bureš J, et al. Pharmacokinetics of the Cardioprotective Drug Dexrazoxane and Its Active Metabolite ADR-925 with Focus on Cardiomyocytes and the Heart. J Pharmacol Exp Ther 2018;364:433-46. [Crossref] [PubMed]
- Hasinoff BB, Herman EH. Dexrazoxane: how it works in cardiac and tumor cells. Is it a prodrug or is it a drug. Cardiovasc Toxicol 2007;7:140-4. [Crossref] [PubMed]
- Bures J, Jirkovska A, Sestak V, et al. Investigation of novel dexrazoxane analogue JR-311 shows significant cardioprotective effects through topoisomerase IIbeta but not its iron chelating metabolite. Toxicology 2017;392:1-10. [Crossref] [PubMed]
- Xie H. Cardioprotective effect of dexrazoxane on gastric cancer patients receiving epirubicin chemotherapy. The Practical Journal of Cancer 2016;31:622-4.
- Li J, Xiao X. A prospective study on the cardioprotective effect of dexrazoxane on breast cancer patients receiving anthracycline containing drugs. Journal of Hunan University of Chinese Medicine 2012;32:48-50+55.
- Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004;56:185-229. [Crossref] [PubMed]
- Wang X, Song H, Tang R, et al. Cardioprotective effect of dexrazoxane to breast cancer patients receiving postoperative epirubicin chemotherapy. Clinical Medicine & Engineering 2016;(11):1473-4.
- Zhao B. The preventive and therapeutic effects of dexrazoxane on the cardiotoxicity induced by pirarubicin. Capital Food Medicine 2017;(06):42-3.
- Lu C, Wang W, Cao S. Cardioprotective effect of dexrazoxane on breast cancer patients undergoing epirubicin chemotherapy. Chinese Journal of Surgical Oncology 2015;(06):368-9+373.
- Liu X, Huang Y. A prospective study on the prevention of the cardiotoxicity induced by pirarubicin. Jiangxi Medical Journal 2016;(03):246-9.
- Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail 2014;16:300-8. [Crossref] [PubMed]
- Roti Roti EC, Salih SM. Dexrazoxane ameliorates doxorubicin-induced injury in mouse ovarian cells. Biol Reprod 2012;86:96. [Crossref] [PubMed]
- Gao L, Li W, Liang J, et al. Study on the effect of dexrazoxane on the breast cancer apoptosis induced by epirubicin. Chinese Journal of Cancer Prevention and Treatment 2013;20:914-7.
- Gao X, Han Z, Du X. Observation of the preventive and therapeutic effect of dexrazoxane on the cardiotoxicity induced by epirubicin. Chinese Journal of Cancer Prevention and Treatment 2010;17:296-8.
- Tahover E, Segal A, Isacson R, et al. Dexrazoxane added to doxorubicin-based adjuvant chemotherapy of breast cancer: a retrospective cohort study with a comparative analysis of toxicity and survival. Anticancer Drugs 2017;28:787-94. [Crossref] [PubMed]
- Guo W, Guan H, Ma A. Systematic review on the effect and economy of dexrazoxane in preventing cardiotoxicity caused by anthracycline chemotherapy in breast cancer patients. Chinese Journal of New Drugs 2017;26:1328-34.
- Huang X, Yang F, Zhang Q. Analysis of off-label drug use prescription of dexrazoxane in dispensary for western medicine. China Health Industry 2017;14:158-60.
- Shaikh F, Dupuis LL, Alexander S, et al. Cardioprotection and Second Malignant Neoplasms Associated With Dexrazoxane in Children Receiving Anthracycline Chemotherapy: A Systematic Review and Meta-Analysis. J Natl Cancer Inst 2015;108:djv357. [Crossref] [PubMed]
- Wang J, Yan Y, Alatangole. Observation and analysis of the preventive and therapeutic effect of dexrazoxane on the cardiotoxicity caused by chemotherapy drugs of cancer. Electronic Journal of Clinical Medical Literature 2016.3:2183-6.
- Zhuang H, Zhang Y, Cai J, et al. Clinical study on the reduction of cardiotoxicity of anthracycline drugs by dexrazoxane combining with Shenmai injection. Chinese Journal of Clinic Oncology 2012;39:348-51.
- Luan X, Gai Y, Wan Y, et al. Observation on the efficacy of dexrazoxane in breast cancer patients treated with anthracycline drugs. Chinese Journal of Evidence-Based Cardiovascular Medicine 2015;7:482-3+486.
- Jirkovský E, Lenčová-Popelová O, Hroch M, et al. Early and delayed cardioprotective intervention with dexrazoxane each show different potential for prevention of chronic anthracycline cardiotoxicity in rabbits. Toxicology 2013;311:191-204. [Crossref] [PubMed]


