Opioids, adjuvants, and interventional options for pain management of symptomatic metastases
Introduction
More than two thirds of patients with metastatic cancer experience pain and a majority of patients with cancer continue to have moderate to severe pain which, regrettably, remains undertreated (1). In fact, the prevalence of cancer related pain is widespread and affects patients with in all cancer stages (1-15). Pain is reported in patients who are on anticancer treatment (16), after curative treatment of their cancer (17-20), and also in metastatic and terminal disease (21). The WHO ladder was established in 1986 and has been validated for pain relief. Characteristics of acute and chronic pain syndromes and methods of assessment of pain are essential to review in order to manage pain. In addition, opioid side effects, adjuvant therapies, and interventional strategies are reviewed in this article.
The WHO ladder
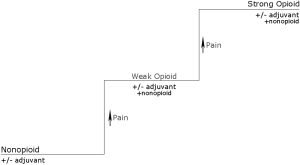
The WHO ladder advocated using a stepwise approach and encouraged using opiates in the treatment of pain while stimulating education regarding the benefits and side effect profiles of these medications. In a 10-year validation study of WHO guidelines for cancer pain relief, the vast majority of patients with prescribers following the WHO guidelines for cancer pain management achieved pain relief and only 12% of patients following WHO guidelines reported ineffective treatment of their cancer pain, demonstrating efficacy and low rates of complications with analgesic therapy according to the WHO guidelines (22). Today, the effectiveness of the three step ladder (Figure 1) is thought to be somewhat lower but even so, pain relief is believed to achievable in a large number of patients (23).
These principles are still used today and have even been adapted to patients who suffer from acute or chronic pain due to a variety of other etiologies (Figure 2).
- Start with oral administration of analgesics. Oral administration is preferable especially if a patient is not restricted by the route of administration of medication;
- Dosing of medications should be given according to pain intensity. Tailor the dosage according to the patient’s pain. Pain relief medications should be given after an assessment of the patient’s pain and highlights the necessity of using a pain scale (24);
- Analgesics should be administered at regular intervals. Medications should be given regularly in accordance with the pharmacokinetics of the medication and pharmacodynamics of absorption and distribution. Medications should not be given on an “as needed” basis and a schedule should be given to the patient and family for adequate pain management at home;
- Treat and carefully monitor for progress and side effects. The patient’s level of analgesia should be closely monitored and common side effects should be addressed including constipation, nausea, and vomiting. The guidelines state that almost all patients receiving opioids will require a laxative but cautions that respiratory depression is rarely seen in patients on chronic opioids;
- Progress should be monitored carefully. Though recommended doses are adequate for initiation, dose titration should be individualized and is highly variable. Doses of non-opioids may have a maximum recommended dose but opioids may be increasingly titrated to achieve pain relief.
The ladder has been revised and updated (25,26) since 1986. Some reviews have advocated eliminating step 2 after evidence that escalating from nonsteroidal anti-inflammatory drugs (NSAIDs) to weak opioids does not necessarily improve analgesia (27). Updates have continued as a result of the continuing evolution and development of newer therapeutic strategies for analgesia. Proposed modifications of the WHO ladder now include an elevator model instead of a ladder model (28) since some advocate going step by step is often ineffective at controlling intense pain. Another adaptation of the original ladder model integrates a fourth step for nerve blocks, spinal administration of local anesthetics, opioids, alpha-2 agonists, spinal cord stimulation, and surgical interventions (24).
Prevalence and characteristics of cancer pain
In cancer patients, a number of causes are commonly associated with cancer-related pain (29-31). One prospective evaluation revealed common etiologies include cancer-related pain from bone (35%), soft tissue (45%) or visceral structures (33%) or otherwise as of an neuropathic origin (34%) (32). Intensity of pain has not been found to correlate with tumor burden and severe pain can occur in all cancer stages (7). Pain can exist as a result of anticancer therapies, comorbidities unrelated to cancer, as well as secondary to underlying cancer itself (32).
A process called nociception result in the neurophysiologic pathway involved in cancer pain. Tissue injury activates nociceptors which are found in skin, muscle, joints, and some visceral organs. Given the complexity of the physiology of pain processes, pain is described as nociceptive, neuropathic, or myofascial (Table 1).

Full table
Pain from nociceptors can be as a result of acute or chronic injury to somatic or visceral tissues. Injury to the bones, joints, or muscles is usually described as “throbbing” or “aching” while visceral pain is characterized as “cramping” or “gnawing” and is poorly localized. Neuropathic pain implies involvement of nerves such as in impingement or inflammation and may occur centrally as well as peripherally. Neuralgia is associated with nerve damage or irritation along a single nerve, e.g., trigeminal. Allodynia refers to pain induced by a non-painful stimulus. Hyperpathia refers to exaggerated pain out of proportion to the stimulus. Dysesthesia is an unpleasant or abnormal sensation in the area of neurologic deficit. Myofascial pain is a muscle pain that occurs in conjunction with other pains such as with neuropathic pain. The trigger point is a localized, highly irritable spot in a taut band of skeletal muscle. The palpation of these trigger points will alter the pain, causing it to increase or radiate. The patient may feel as if they are having a muscle spasm.
Acute and chronic pain syndromes
Acute pain is associated with increased activity in the sympathetic nervous system and may result in diaphoresis, tachycardia, and hypertension. However, tolerance to sympathetic activity can develop quickly as the pain becomes chronic. Acute pain syndromes have a variety of causes (Table 2) that may be disease-related complications (e.g., obstruction from neoplasm, pathologic fracture) but may also be secondary to procedures and antineoplastic treatments (radiation mucositis, neuropathy) (33).
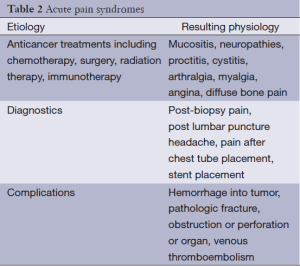
Full table
Overt pain behaviors manifest in acute as opposed to chronic pain syndromes and include grimacing, moaning, and splinting. The direct effects of malignancy as well as effects of anticancer therapies can contribute to chronic pain (Table 3) (33). Chronic pain that remains unrelieved can result in depression, insomnia, anxiety, anorexia, and decrease in quality of life.
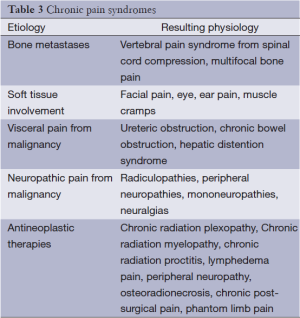
Full table
Assessment of pain including pain scales
Adequate assessment of pain is essential for effective pain management. Obtaining a thorough history and physical examination is the beginning of obtaining a thorough pain history. Components of the pain history include location of the pain, onset (acute versus chronic), exacerbating and relieving factors, quality (sharp, dull, throbbing, gnawing), radiation, associated symptoms, severity, and temporal pattern (continuous, intermittent), all of which can help to determine a possible cause. Past history of medications with successful or unsuccessful outcomes should be elicited including prescription, over-the-counter, and home remedies. Prior instances of pain, date of onset of the current episode, length of duration, and trajectory of improvement or worsening should be recorded. A detailed history can give clues to cancer progression, recurrence, and complications.
The patient interview
There are several steps to comprehensive pain assessment. Firstly patients may be hesitant to admit to feeling pain. If this occurs, use other words than pain, such as discomfort to help them describe what they are experiencing. When a patient’s mental status interferes with their ability to communicate their pain, get information from family members or other caregivers.
It is not unusual in chronic pain for an individual not to remember when the pain began, since it has usually been there for an extended period of time. It is important to find out the location, duration, severity, and characterization of the pain such as crampy, throbbing, burning, or shooting pain. Severity can be measured in many ways including numerical scales such as a 0 to 10 scale, or 0 to 5 scale, or simply mild, moderate, or severe. There is a scale used with children which involves looking at faces to describe level of pain. This can be used with the elderly and those who are severely cognitively impaired.
Besides asking about the severity of pain, it is important to try to determine what level is tolerable. In addition, sometimes having the patient draw the location of pain on a picture of a person, which can be made large for the visually impaired, is the most helpful part of the interview.
Assessment of aggravating and relieving factors for the pain is important. Many individuals with pain have tried things that relieve their pain, and may help the clinician find other complementary modalities to help relieve the pain. As an example, if a patient finds that rubbing the area helps relieve the pain, modalities such as massage and use of a transelectrical nerve stimulator may be useful in the pain regimen.
When questioning the patient and or the family about the pain, it is important to understand how the pain has affected physical and social function. Clearly, the higher level of pain will usually lead to a decrease in physical and social function. Indeed in any patient, a decrease in physical or social function can many times be the major clue that the individual is having pain. Lastly it is important to ask about associated symptoms that are sometimes seen at the same time as pain.
Observing the patient
After questioning the resident the second step in the assessment process is observing the resident’s behaviors. It is important to observe for moaning, crying, groaning, sighing, wincing, frowning, grimacing, protecting, guarding, rubbing, or favoring an area of the body. Other things to observe for include lying very still, decrease in usual activities, and refusing to eat. Other indications of pain may be changes in mood or signs of depression such as sleeping problems, sadness, anxiety, anger, and decrease in participation in social activities. Changes in social contacts and relationships such as arguments with family or friends, less contact with family or friends, or striking out at caregivers, physically or verbally, may be an indication that the patient is experiencing pain.
Pain assessment in the cognitively impaired patient
Cognitive impairments are varied and may include changes in memory (forgetfulness), attention (difficulty focusing; becoming sidetracked), language (inability to find the right words), orientation (confused about time and place), calculations (difficulty adding numbers), and visual-spatial skills (difficulty reading or understanding chart or graph).
There are many measures that exist which measures pain intensity, however few have been well validated in the cognitively impaired and elderly patient. The pain thermometer, which is a modified verbal descriptor scale, has been found to be effective in the elderly, even those with moderate cognitive impairment (34). The pain map, or drawing, of the person (found in the McGill Pain Questionnaire) has also been found to be reliable for the elderly (35). The numerical graphic rating scales, are often problematic in the elderly resident (34). The Faces Pain Scale has been shown to have good test-retest reliability and construct validity in older adults (36), however it has not been adequately tested in those with moderate to severe cognitive impairment. When patients are too cognitively impaired to report their pain using the traditional measurement tools, it is necessary to observe for behavioral manifestations of pain, as discussed above.
Reassessment of pain
Reassessment of pain is at least as important, as the initial assessment of pain. Reassessment of pain for the patient with cancer needs to be done any time there is a new pain, any time there is an unacceptable level of pain, any time there is a procedure that may produce pain such as intravenous sticks, dressing changes, and bone marrow biopsies, and after an intervention which is done to help alleviate the pain such as after receiving opiates. Items to be reassessed include improvements or worsening of conditions, treatment side effects, compliance problems, such as not taking medications as scheduled, functional improvement, and effectiveness of other treatment measures such as physical therapy, music, art, and training in coping skills.
Psychosocial assessment
The psychological assessment can be potently influenced by the clinicians approach. The essential component to a valid psychosocial assessment is for the health care provider to truly engage in active listening and maintain an unbiased demeanor. Establishing a therapeutic relationship is built upon with each interaction. Building an alliance in the plan of care with the patient and family will enable the patient to best participate in decision making, especially in the light of new onset of symptoms and advancing disease or poor prognosis.
Eliciting an accurate psychosocial assessment requires the clinician to have knowledge of society expectations and for personal self-assessment. Many psychosocial and spiritual issues fail to be recognized by clinicians and with initial labeling or categorizing the patients’ true inner experience may be unappreciated, thus untreated.
Clinicians should assess their own style when speaking to patients about pain. Eye contact suggests confidence and optimism and will assist in moving the trust factor forward. Incorporating a larger oncology team may initially be intimidating during the assessment process. The psychosocial assessment should agree with the physical assessment in terms of communication. Nonverbal clues will give you guidance in how soon you can broach sensitive subjects. Initial and ongoing re-assessment can provide an opportunity for disclosure and focus on fears and anxieties of the patient and family. Incorporating faith and the patient’s greater spiritual community are essential. The significance of the patient’s cancer pain and the significance of an individual’s belief system on ones’ disease are all important questions. Table 4 lists additional appropriate questions to ask.
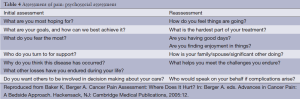
Full table
Coping, anxiety, depression
The diagnosis of cancer equates to a cascade of perceived loss of control in all areas of the patients life. Normalcy, whatever that has been for the patient and their family, will now be a bench-mark to maintain. Coping strategies may be helpful to destructive. Denial, anger, avoidance, regression, rationalization, intellectualism are all possible forms of psychological presentation to the clinician. It is critical to assess effective coping mechanisms for this patient now or harmful to him or others? If there is denial or other areas of concern, ask “what if” questions to probe a little deeper. If it is acceptable, then leave the coping behavior intact. Past, present, and potential future losses are broad and include unresolved grief, e.g., from divorce, death of a parent, death of a pet, role changes within the family and community, and changing resources, e.g., finances, job, physical energy.
Anticipatory anxiety and pain are under-explored in the assessment process. They are not readily divulged and may only be declared at the peak of the anxiety, i.e., panic attacks while in the MRI, or perceived over use of a PCA. Inquiring about negative, past incidents will be an opportunity to prevent and plan for easier future treatments. Clinically, signs and symptoms of anxiety are apprehension, fear out of proportion to event, limited concentration and changes in motor tension.
Functional abilities and pain are directly correlated with depression. Differentiating a depressed mood as adjustment reaction or clear clinical depression may require a referral to a psychiatrist. The majority of cancer patients who have suicidal ideation have an underlying psychiatric disorder or poorly controlled symptoms. Examples from the DSMIV criteria for clinical depression include persistent depressed mood for most of the day for greater than 2 weeks, change in appetite, sleep disturbance, decreased energy, decreased attention, and suicidal thoughts.
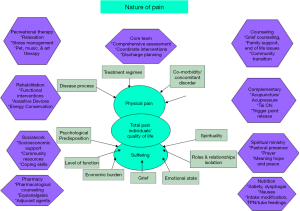
Existential pain is synonymous with suffering or psychic pain. The experience of pain is profoundly affected by mortal angst, which in turn is influenced by premorbid personality, coping mechanisms, as well as psycho-social-spiritual support systems. Pain of this nature is not responsive to opioid manipulations, is just as “real”, and is therefore more difficult to manage. If this very significant part of a patient’s experience is avoided, the soul searching questions (and possible resolutions) which arise when an individual faces his/her mortality are neglected. Utilizing counseling, spiritual ministry or religious support, in conjunction with creative modalities of art, music, recreational therapies, labyrinths, pet therapy, and healing touch, may greatly assist an individual who is preparing for the reality of the struggle ahead, whatever it may be. Total pain (Figure 1) is the aggregate of all types of pain that an individual experiences. In order to obtain relief, one must treat all natures of pain and suffering.
Management of cancer pain with opioids
Weak opioids including hydrocodone, codeine, and low-dose oxycodone compose step 2 of the WHO ladder. These opioids are usually weak since they are put in combination with a nonsteroidal or acetaminophen. Mild or moderate pain is addressed with these agents as well as other mu receptor agonists such as tramadol. For cancer related pain, escalation from step 1 to 2 of the WHO ladder does not necessarily improve analgesia (27). An additional limitation of weak opioids is the “ceiling effect” for which increasing dose above a threshold does not increase analgesia. Effectiveness of the second step of the WHO ladder has a time limit of 30-40 days after which insufficient analgesia is achieved and a shift to the third step is begun (37).
Step 3 of the WHO ladder consists of strong opioids. For many years, morphine has been the opioid of choice for moderate to severe cancer pain. More recently, oxycodone and hydromorphone have been recommended as first line opioids for cancer pain (38). Other strong opioids include fentanyl, high-dose oxymorphone and methadone. A combination of long and short-acting opioids is generally recommended for chronic cancer pain. Long-acting opioids are used for chronic baseline pain and short-acting opioids are used for breakthrough pain and may require repetitive, frequent dosing. Long-acting opioids include methadone as well as extended-release morphine, oxycodone, oxymorphone, or hydromorphone. A comparison of selected opioids is listed in Table 5.
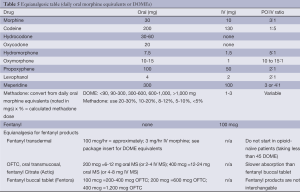
Full table
In treating continuous pain, around-the-clock dosing should be used. Normal release opioids such as intermediate-release morphine, oxycodone, or hydromorphone should be used every 4 hours while sustained-release morphine, oxycodone, or hydromorphone should be used every 12 hours.
Breakthrough pain is defined as an abrupt, short-lived, and intense flare of pain in the setting of chronic pain successfully managed with opioids. Breakthrough pain is common and is reported in 50-70% of cancer patients (39). Current recommendations advocate rescue dosing at 25-50% of the 4 hourly dose or 10-20% of the total daily opioid dose. Suboptimal pain intervention results in end-of-dose failure and can be improved by titrating the around-the-clock dose rather than shortening the dosage interval (40).
Dosing should be individualized according to age, body habitus, drug interactions, and organ system dysfunction. In mild hepatic impairment, morphine and hydromorphone are relatively safe but should have a reduction in dose in severe hepatic dysfunction. In renal failure, methadone, fentanyl, buprenorphine are acceptable with few dose adjustments necessary. Oxycodone elimination is affected by both hepatic and renal failure. Opioids that are metabolized through the CYP450 system are vulnerable to drug interactions including fentanyl, oxycodone, and methadone. Glucoronidated opioids like morphine and hydromorphone are less likely to be problematic.
When treating with opioids one needs to think about the definitions of tolerance, physical dependence, addiction and pseudoaddiction (Table 6).

Full table
Side effects of opioids
Side effects of opioids are common prior to achieving pain relief (Table 7). Side effects of opioids include nausea and vomiting, constipation, sedation, confusion, myoclonus, and pruritis, many of which may be managed symptomatically. The most common dose-limiting side effects are constipation and confusion. Marked variability exists between individuals in terms of adverse effects which are due to differences in age, comorbidities, genetic variables, and interactions with other medications. Managing side effects increases the likelihood of effective pain relief through improved adherence. Strategies for the treatment of side effects include symptomatic management, dose reduction, and switching or rotating opioids.
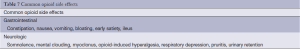
Full table
Gastrointestinal (GI) side effects
Opioids affect gastrointestinal motility commonly. Constipation, bloating, early satiety, and pain are all possible. Occasionally, ileus and abdominal pain can result. Narcotic bowel syndrome is a term applied to pain that is significant and a result of bowel manifestations in patients treated with opioid therapy (41).
Among the most common and persistent side effect is constipation which is present in 10-15% of patients undergoing opioid treatment (42). Among the factors that contribute to its development include the binding of opioid to receptors in the GI tract and central nervous system that reduce motility via anticholinergic and direct mechanisms, excessive water and electrolyte loss from feces resulting from increased GI transit time, and concurrent conditions including the use of other constipating drugs, dehydration, immobility, metabolic abnormalities (e.g., hypercalcemia), chemotherapy (e.g., vinca alkaloids), and direct obstruction by tumor of the GI lumen. Two recent reviews found that less constipation could be achieved with transdermal fentanyl than oral sustained release morphine (43,44).
Prevention of GI side effects may be achieved with dietary modifications including increased consumption of fluids and dietary fiber. However, if bowel obstruction is suspected, the patient is debilitated, or hydration is difficult to maintain, fiber should be discontinued since introduction of fiber may worsen obstructive symptoms. Prophylactic laxative therapy should be started upon initiation of opioids with senna two tablets at bedtime with or without a stool softener (e.g., docusate 100 mg twice daily) or osmotic laxative (e.g., lactulose 30 mL daily, miralax). If no clear precipitant can be found for constipation including no recent increase in opioid therapy, an assessment of alternative or contributory causes should be undertaken and when necessary, imaging studies and colonoscopy. Patients who have not passed stool in several days may require disimpaction if enema fails to clear the rectal vault. Once impaction has been cleared or ruled out, laxative therapy may be reinitiated.
Management of refractory constipation include methylnaltrexone, a peripherally acting opioid antagonist that does not cross the blood brain barrier and therefore does not induce symptoms of opioid withdrawal (45,46). The efficacy of methylnaltrexone is approximately 50% (47). Methylnaltrexone is given subcutaneously every other day and its dosing frequency can be increased but not to exceed once daily. Higher than approved doses may provide additional benefit as a subgroup of patients received dose escalation and their success rate for achieving a bowel movement was 24% (compared to 15% at the lower dose). Concerns for severe abdominal pain and bowel perforation with methylnaltrexone in patients with advanced cancer have led to an FDA-issued warning to physicians to use caution when administering this drug to patients with known of suspected lesions in the intestinal wall.
Oral naloxone has also been used to treat constipation (39,48). Given that it reverses systemic opioid effects, it may increase pain or induce withdrawal.
Another orally administered peripherally acting mu receptor antagonist is alvimopan, approved in the US after positive results in a double-blind randomized trial with 518 participants, patients were randomized to alvimopan 0.5 mg daily, twice daily, or placebo (49). The twice daily intervention group had significant improvement in the proportion of patients experiencing three or more spontaneous bowel movements with no laxative use in the prior 24 hours (72% in twice daily intervention group versus 48% of patients taking placebo).
Somnolence and mental clouding
Symptoms of somnolence or mental clouding can wane over a period of days or weeks and can be persistent. Cognitive impairment ranges widely from slight inattention to disorientation, severe memory impairment, extreme contusion, and delirium. Hypnagogic illusions and hallucinations may occur as well as mood disturbances that are more often dysphoric than euphoric. Risk factors for cognitive dysfunction include diagnosis of lung cancer, daily opioid doses of 400 mg or greater, older age, low performance status and time since cancer diagnosis less than 15 months (50).
Management includes eliminating or reducing centrally acting medications that are extraneous while addressing other etiologies (e.g., metabolic disturbances, dehydration, other drugs). If analgesia from the opioid regimen is satisfactory, an empiric dose reduction of 25% may provide beneficial results. However, if cognitive dysfunction prevents effective analgesia, opioid rotation may be tried. Psychostimulants including methylphenidate (51), modafinil (52), dextroamphetamine (53), and caffeine (54) have been used. Sympathomimetic side effects of these psychostimulants may be problematic and therapeutic effects of psychostimulants sometimes wanes over time. Relative contraindications to the use of psychostimulants include preexisting anorexia, severe insomnia, anxiety, paranoid ideation, significant cardiac disease, or poorly controlled hypertension.
Nausea and vomiting
Nausea is frequently seen at initiation of therapy but persistence of nausea is not commonly seen. Opioids may cause nausea via three mechanisms including a direct effect on the chemoreceptor trigger zone, enhanced vestibular sensitivity, and delayed gastric emptying.
Opioid dosages that have been gradually increased rather than rapidly may prevent nausea. If persistent nausea occurs it is likely in the context of other gastrointestinal systems (e.g., anorexia, early satiety, abdominal bloating). Chronic nausea generally responds to drug therapies for acute nausea (55) including dopamine antagonists [e.g., prochlorperazine, metoclopramide (56)] and serotonin receptor antagonist [e.g., ondansetron (57)]. Risperidone was shown in a small observational study to decrease refractory nausea and vomiting due to opioids in advanced cancer patients (58). Opioid rotation may also be considered and has been shown to significantly result in less nausea and vomiting in two studies. Switching from oral to subcutaneous route produced significantly less nausea and vomiting (59,60). Other medications that may help nausea and vomiting include scopolamine patch and meclizine. Acupressure bands and acupuncture may also be helpful.
Myoclonus
Thought the etiology may be multifactorial from drugs and metabolic derangements, uncontrollable spasms, termed myoclonus is common and dose-related in opioid therapy. Data was insufficient to affirm benefits of any drug for its management (61) but a low-dose benzodiapine [e.g., lorazepam (62)] may be considered or a change to an alternative adjuvant analgesic to help in reduction of the opioid dose.
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia is a paradoxical response where patients may become more sensitive to certain painful stimuli. They may experience pain from non-painful stimuli (allodynia) and the phenomenon is linked to analgesic tolerance. When suspected, opioid rotation may be an option (63).
Respiratory depression
When therapy is administered according to guidelines, respiratory depression is uncommon but may be seen when rapid increase of opioids is necessary for pain control. In the setting of sleep apnea or other serious cardiopulmonary comorbidity or when combined with another sedative-hypnotic, the risk of respiratory depression is increased. Careful selection of initial dose and conservative uptitration is necessary.
Management with naloxone should be given only in cases of symptomatic respiratory depression or for progressive obtundation. Naloxone can be given in a small bolus injection of dilute solution and repeated doses may be necessary as its half-life is shorter than most opioids. Naloxone should not be given to somnolent but easily arousable patients. Alternatively, withholding further opioids until respiratory rate improves or pain returns should be considered.
Pruritis
Pruritis from morphine is thought to cause histamine release from mast cells although other opioids (i.e., fentanyl, oxymorphone) are less likely to produce histamine release but are assocated with pruritis, the mechanism of which is uncertain (64). Despite uncertainty, antihistamines are commonly used as first-line agents for opioid-induced pruritis. Low doses of opioid antagonists are effective treatment for pruritis in patients with non-cancer pain receiving short-term opioids, however interventions in patients receiving long-term use of opioids has not been investigated. Paroxetine according to some anecdotal experience suggests benefit (65). Some evidence exists for use of low doses of opioid antagonists including nalmefene and nalbuphine in the postoperative setting but opioid induced pruritis in long-term use of opioids have not been studied (66).
Urinary retention
Urinary retention is a result of opioid-induced effects on peripheral nerves that innervate the bladder by increasing the tone of the sphincter and binding to spinal receptors causing total bladder relaxation (67). Initial management consists of catheterization of the bladder and an effort to reduce drugs that could contribute to urinary retention, e.g., anticholinergics.
Other drugs effective in reversing urinary retention include naloxone which also has been shown to reverse analgesia postoperatively (68). Anecdotally, nalbuphine (69) and alpha-1 blockers such as tamsulosin can be used to treat urinary retention in some part related to prostatic hypertrophy.
Adjuvants
Adjuvant drugs are sometimes necessary in order to control the many facets of pain. This tenet recognizes that pain is sometimes multifactorial. Patients may require antidepressants if they remain depressed despite pain control, anxiolytics if the patient remains anxious, as well as corticosteroids, neurolytic, and neurosurgical blocks. The term “adjuvant” initially referred to drugs that were marketed for indications other than pain but were found to be useful in those receiving analgesia with opioid therapy. The diversity of these drugs has increased dramatically in number in the past few decades.
If and when a patient is unable to achieve adequate analgesia without dose-limiting side effects, a number of things can be considered including optimizing opioid therapy including adjusting the dose or rotating to a different opioid. The addition of an adjuvant analgesic may also be considered after the opioid dose is optimized. Available adjuvant analgesics include multipurpose analgesics that are used for any type of pain, those used for neuropathic pain, those for bone pain, and those for pain in the setting of bowel obstruction (69-72).
Multipurpose analgesics
These medications have broad analgesic efficacy and include glucocorticoids, antidepressants, alpha-2 adrenergic agonists, cannabinoids, and topical therapies.
Glucocorticoids alleviate symptoms of pain, nausea, fatigue, anorexia, and malaise. In addition, they may be beneficial in neuropathic pain, bone pain, pain secondary to bowel obstruction, duct obstruction, pain caused by lymphedema, and headache caused by elevated intracranial pressure. Most likely, glucocorticoids act to reduce tumor-related edema and have anti-inflammatory effects as well as direct effects on nociceptive neural systems. Dexamethasone is a first-line agent due to its long half-life and relatively low mineralocorticoid effects. Prednisone and methylprednisolone are also acceptable. Long term toxicity can include myopathy, immunocompromise, and adrenal insufficiency. Usually these toxicities are mitigated by limited life expectancy. High-dose regimens have been shown to be more effective in relieving pain than lower dose regimens in spinal cord compression (73). However, limited evidence and the potential for dose-related toxicity should be considered when prescribing these drugs.
Antidepressants have been widely studied in patients with chronic pain although relatively few studies have included cancer patients. They are especially effective in the setting of neuropathic pain. The mechanism of action is thought to be enhanced availability of monoamines, decreased norephinephrine reuptake, as well as increased serotonergic and dopaminergic effects in the synapses of the descending pain modulating system. Analgesic efficacy is best established with tricyclic compounds, serotonin-norepinephrine reuptake inhibitors (SNRIs). First line analgesic antidepressants in patients with cancer would be a tricyclic (e.g., desipramine starting at 10-25 mg at night) or a SNRI (e.g., duloxetine at 20-30 mg daily) and the choice may be determined by an individualized assessment of risk and cost. Evidence also exists for efficacy of serotonin-selective reuptake inhibitors (SSRIs), although these would not be first-line and bupropion (75 mg twice daily), a dopamine reuptake inhibitor. Dosages may be titrated upwards while monitoring for intolerable side effects (74,75).
In terms of side effects, tricyclic compounds are relatively contraindicated in patients with severe heart disease, prostatic hypertrophy, and narrow-angle glaucoma. Side effects of SNRIs include nausea, sexual dysfunction, somnolence, and mental clouding. With up-titration of desipramine, the patient should be monitored for prolongation of QTc interval and the development of cardiac arrhythmias. While duloxetine and buproprion do not prolong the QTc interval, duloxetine has gastrointestinal side effects including nausea, dry mouth, and constipation and buproprion causes jitteriness and headache.
Alpha-2 adrenergic agonists include clonidine and tizanidine. These agents can provide relief in diverse types of pain. Spinally administered clonidine has been shown to be particularly effective in neuropathic pain relief (76). The mechanism of action of these drugs may be related to increased activity of monoamine-dependent, endogenous pain modulating pathways. Side effects of alpha-2 adrenergic agonists include somnolence, dry mouth, and hypotension.
Cannabinoids are derived from the cannabis plant and the primary psychoactive agent is delta-9-tetrahydrocannabinol (THC) or dronabinol which serves to activate receptors in both the peripheral and central nervous system. Some evidence shows efficacy in cancer patients (77) although concern regarding their abuse potential has slowed their development. In Canada, nabiximols or sativex is an oromucosal spray containing THS plus other cannabinoids for treatment of neuropathic pain from multiple sclerosis and is also used in cancer patients. In this double-blind trial, 177 cancer patients suffering from ineffective analgesic control of pain symptoms, nabiximols was up titrated and the adjusted mean pain score was significantly reduced in the group taking nabiximols compared to the placebo group though some bias could have been present in terms of differences in baseline opioid dosing in the two groups (77). Nabilone is another cannabinoid that would be considered for cancer patients whose pain is refractory to opioids and after a trial of other adjuvants. No controlled studies address the efficacy of inhaled marijuana as an adjunct to chronic pain in cancer patients. The side effects from cannabinoids are most commonly dizziness, somnolence, and dry mouth. Despite legalization in the Netherlands, Canada, and several states, in the US, cannabinoids is still illegal at the federal level.
Topical therapies
Benefits of using topical therapies as an adjunct to other analgesic include the ability to directly deliver analgesia to the site that is presumably responsible for persistent pain. Lidocaine patches are transdermal patches that can be used for regional pain of all types. Patients with postherpetic neuralgia (67), diabetic neuropathy (78), and osteoarthritis (79) revealed positive impact on pain. Most frequent adverse effects include skin irritation. Other local anesthetics include EMLA or eutectic mixture of local anesthetics cream (80), capable of producing dense local cutaneous anesthesia, capsaicin (80), a naturally occurring compound of the chili pepper, and other drugs that have been compounded into creams for patients with pain including ketamine and gabapentin.
Neuropathic pain
Drugs especially appropriate for relieving neuropathic pain include the analgesic antidepressants and anticonvulsants. Duloxetine was shown to have improved quality of life scores, and decreased numbness and tingling in patients with painful chemotherapy induced peripheral neuropathy in a multi-institutional trial conducted by the Cancer and Leukemia Group B (81). There is some evidence for anticonvulsant analgesics effects with gabapentin and pregabalin (82-84). Both these drugs act by binding to alpha-2 delta protein modulator of N-type voltage-gated calcium channels which reduced calcium influx into the neuron and therefore lessens depolarization. They are both excreted by the kidneys and dose reduction is necessary in patients with renal dysfunction. Somnolence, mental clouding, and dizziness are main side effects although edema and weight gain occur less frequently. Gabapentin has saturable kinetics at doses of higher than 1,800 mg per day and therefore has a pharmacokinetic “ceiling” whereas pregabalin absorption is not saturable and therefore maximum therapeutic dosing is less predictable. The starting dose of gabapentin is usually 100 to 300 mg per day while pregabalin is 50-75 mg daily in two divided doses. Tapering these drugs is preferable in the setting of discontinuation.
Numerous other anticonvulsants have potential analgesic effects including carbamazepine, valproate, and phenytoin, however data are limited. Oxcarbazepine, a metabolite of carbamazepine has similar anticonvulsant properties and a safer pharmacologic profile and has been shown to be effective in trigeminal neuralgia (85).
Other drug classes that have some activity for neuropathic pain include tizanide, dronabinol, and topical agents. A brief intravenous infusion of lidocaine has been shown to give prompt pain reduction in severe neuropathic pain (86). Ketamine, a NMDA receptor antagonist, has been used at subanesthetic doses for severe refractory pain (87) and earlier evidence suggested enhanced control of cancer pain when given with morphine (76,88,89), however later trials failed to demonstrate added analgesia (89-91) and a 2012 Cochrane review (92) concluded that the evidence was insufficient to assess the effectiveness of ketamine. Regardless of lack of high quality evidence, experts still treat refractory neuropathic pain with ketamine using mainly anecdotal evidence. Still, delirium and psychotomimetic effects of ketamine are problematic and gradual dose titration may help to reduce some of these adverse effects (93).
Bone pain
Patients with bone pain may look to radiation therapy for painful bone metastases as well as kyphoplasty and other spinal surgeries designed to treat pain from malignant spinal fractures. However, medications can be used to treat pain in patients with metastatic bone disease as well and include glucocorticoids like dexamethasone, osteoclast inhibitors like bisphosphonates, and bone-seeking radionuclides (Table 8).
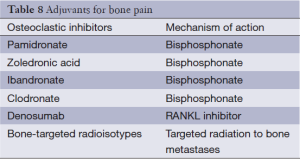
Full table
Osteoclast inhibitors include bisphosphonates, the use of which is supported by significant data. These drugs act by directly inhibiting osteoclastic activity, causing apoptosis of osteoclasts, and also stimulating osteoblastic activity. Head to head trials comparing the bisphosphonates are few so choice of bisphosphonate is left to patient and clinical preference and convenience. Generally, these drugs are well tolerated. Complications may include renal dysfunction, flu-like illness, hypocalcemia, and osteonecrosis of the jaw.
Donosumab is a monoclonal antibody targeting receptor activator of nuclear factor kabba B ligand (RANKL), a key component in the pathway for osteoclast formation and activation. Progression of pain was decreased in those receiving donosumab when compared to zoledronic acid in patients with advanced breast cancer and bone metastases (94). In addition, denosumab is not associated with renal dysfunction or with flu-like syndromes, making it a viable alternative for patients who have encountered these adverse effects while using bisphosphonates. However, the cost of denosumab is substantial compared to bisphosphonates (95).
Bone targeting radionuclides include strontium-89 and samarium-153 which selectively deliver radiation to bone metastases. Often used for men with metastatic prostate cancer, this approach is for patients with refractory multifocal bone pain.
Bowel obstruction
Many patients with advanced intraabdominal or pelvic tumors have inoperable bowel obstruction and are not appropriate for a stent. In these patients, decompression of gastric contents can be achieved with nasogastric tube or percutaneous gastrostomy tube and management with IV fluids has been conventional approaches. However, control of pain, distention, and vomiting using medical management may include use of glucocorticoids to decrease edema surrounding the tumor and use of octreotide and anticholinergic agents to lessen intraluminal secretions and peristaltic movements. Octreotide, dexamthasone, and hyoscine butylbromide are effective at reducing symptoms of bowel obstruction according to one review (96). Scopolamine and glycopyrrolate are also anticholinergic drugs that reduce gut motility and decrease secretions.
Interventional therapies
Though optimization of opioid therapy and use of adjuvants results in a balance of satisfactory analgesia in many cancer patients, a substantial number unfortunately do not achieve effective pain relief through the medications described above. Interventional pain management strategies include a diverse group of invasive therapies: injections, nerve blocks, implanted neurostimulation, and neuroaxial drug infusion techniques. Evidence for these therapies remain limited, most of which are implemented by those who have received specialized training.
Injection therapies
Injections into the soft tissue and joints are commonly to address focal musculoskeletal pain and myofascial pain, or pain with distinct trigger points. Injections can be given epidurally, into facet joints, and into the sacroiliac joints for those with and without cancer related pain.
Vertebroplasty and kyphoplasty
Developed to address pain from vertebral collapse in neoplastic disease to the spine, vertebroplasty is the percutaneous injection of bone cement or methylmethacrylate under fluoroscopic guidance into a collapsed vertebral body while kyphoplasty involves the introduction of inflatable bone tamps, termed balloon kyphoplasty, that restore the height of the vertebral body. Pain reduction ranges from 47-87% with vertebroplasty (97) and kyphoplasty was associated with not only pain relief but improved functional outcome and early improvement in vertebral height loss and spinal deformity but these benefits were not maintained (98). Cancer patients have greater quality of life and sustained improvement in activity over 12 months. However, they also had non-Q wave infarction attributed to anesthesia and cement leakage to the disc as adverse events (99).
Direct head-to-head trials with vertebroplasty compared to kyphoplasty are limited. For patients with multiple myeloma and bone disease, pain may be refractory to bisphosphonates, calcitonin, and other opiates. Guidelines suggest balloon kyphoplasty rather than vertebroplasty for those patients with symptomatic vertebral compression fractures from lytic metastases (100).
Neural blockade
The term nerve block refers to delivery of an agent or device that modulates nociceptive afferent input to the central nervous system with either a nondestructive analgesic or a neurolytic drug. A diagnostic nerve block is performed to determine the afferent terminal of the noxious stimulus and can be performed using local anesthetic blocks which affect both sensory as well as motor neural activity. A diagnostic nerve block can be helpful to clarify if the pain is maintained using sympathetic neurons or transmitted via nociceptors travelling with sympathetic nerves. A prognostic nerve block is a neurolytic or neurodestructive block that extends relief of pain through the destruction of the nerve and sensory loss. Usually, a local anesthetic nerve block is performed before the neurolytic block to determine if the sensory loss is satisfactory. A therapeutic nerve block is applied to an injection to address the underlying etiology of the patient’s pain and include epidural steroids for an acute herniated disc and epidural blood patches for a post-spinal tap headache, both of which occur in cancer patients as well as patients without cancer.
Non-neurolytic blocks
A block performed using bolus or continuous infusion of local anesthetic is termed non-neurolytic blocks and address pain that is either acute or chronic. Transitory interruption of the afferent nociceptive input may result in pain relief that outlasts the duration of effect of the anesthetic. In fact, repeated local anesthetic blocks can be a therapeutic strategy towards relieving pain. Delivery of non-neurolytic blocks may be via catheter into the epidural space, to a peripheral nerve, or nerve plexus. Patients with focal regional pain syndrome may achieve good pain relief with infusion of local anesthetic especially if they have demonstrated prior benefit from bolus injections. Epidural catheters with continuous anesthetic infusions can be performed for those with larger areas of pain and may be combined with opioids. Case reports have demonstrated anecdotal evidence that such blocks are effective in cancer pain (101,102).
Sympathetic nerve blocks
When pain involves somatic structures, the choice of a sympathetic rather than somatic nerve block may be done if the perception of the pain is thought to be maintained by sympathetic efferent activity. For example, complex regional pain syndrome is likely characterized by sympathetically-maintained pain. Clues to characterize sympathetically-maintained pain include patients with focal autonomic dysfunction such as vasomotor instability, sweating, increased hair growth, and thinning of the skin. In this case, a trial of sympathetic block may be considered.
Many cancer patients often have difficult to control visceral pain. Sympathetic nerve block is especially appropriate for visceral cancer pain which is often at least somewhat manageable with sympathetic blockade. Blockade of the sympathetic nerve interrupts both the efferent sympathetic and afferent fibers traveling in the same nerve.
Sympathetic blocks are targeted to the anatomic location of the sympathetic nervous system and result in anesthesia of their respective sympathetic nerves (Table 9).

Full table
Somatic nerve blocks
These nerve blocks can be done for cancer patients and those without cancer. Both bolus and continuous epidural anesthetic infusions may be used to denervate specific areas of the body. For example, a nerve block at the brachial plexus can denervate the shoulder or arm, a Gasserian ganglion block can be used to denervate part of the face.
Neurolytic blocks
Neurolytic techniques destroy afferent neural pathways including somatic and sympathetic efferents and employ surgery, cryotherapy, radiofrequency thermal coagulation, or chemical neurolysis using alcohol or phenol. Traditional methods for neurolytic nerve block include phenol and alcohol. Newer approaches include nondestructive analgesics. Given neurolysis results in destruction of the nerve, regeneration may occur if axolemma are intact after 3-6 months and can lead to functional recovery of the nerve. After neurolysis, deafferentation pain syndrome may manifest as an adverse effect and may be as difficult to treat as the initial pain syndrome. For this reason, neurolysis is considered a last resort therapy. Therapeutic strategies for neurolysis include the celiac plexus for pain originating from upper abdominal malignancy, especially pancreatic cancer. Superior hypogastric plexus neurolysis may be considered for visceral pelvic pain refractory to opioid interventions.
Implanted neurostimulation and neuroaxial infusion
Implanted catheters may be used to deliver infusions of local anesthetic for analgesic purposes. These strategies avoid side effects associated with systemic medications but adverse effects include infection and the possibility of mechanical failure.
Implanted neurostimulation
Dorsal column stimulation is the most common type of implanted neurostimulatory treatment. An electrode is passed into the epidural space at the level of desired analgesia. When an electrical current is applied, paresthesias and sometimes analgesia localized to the region of pain provide relief. Overall, analgesic use is reduced when using spinal cord stimulation when treating refractory cancer-related pain (103). Those patients with debilitating cancer pain of neuropathic origin and who are at the end of life are likely the most appropriate candidates for spinal cord stimulation.
Neuraxial infusion
Infusion of drugs into the epidural or intrathecal space is termed neuraxial infusion. If the patient is likely to survive for months, a catheter can be tunneled under the skin and can deliver medications via an external pump or a fully implanted infusion pump. However, it the patient’s survival is expected at days to weeks, a percutaneous catheter with an external pump may be most appropriate.
Epidural drug delivery necessitates higher drug doses but permits delivery to fewer dermatomes while intrathecal infusion delivers far less drug to achieve a comparable level of anesthesia. The frequency of pain relief, however, is similar with both strategies (104). Morphine, targeting receptors in the dorsal horn, and bupivicaine, which inhibits sodium channels in spinal nerve roots, have been used commonly in neuraxial infusions and work synergistically to relieve neuropathic pain (105). Epidural clonidine can be added to morphine infusions and can be effective in some patients with neuropathic cancer-related pain (106) and intrathecal baclofen is sometimes used as well. In addition, ziconotide, a neural N-type calcium channel blocker, can be used in those patients who are refractory to intrathecal morphine treatment for cancer pain.
Intrathecal drug administration has shown better pain reduction (at least a 20% reduction using visual analog score) when compared to patients with medical management for their cancer pain. Patients had less fatigue, depressed level of consciousness, and a trend toward higher survival rates at 6 months. In addition there was a trend towards improved survival at six months (107). Risks of indwelling catheter lines include infections like epidural abscess and meningitis, bleeding, respiratory depression especially in those patients who are opioid-naive, blockade of both sensory nerves causing unpleasant numbness and proprioceptive, motor blockade causing weakness, sympathetic blockade causing hypotension. Complications can be minimized with low initial drug dosing and careful uptitration with close monitoring for potentially life-threatening effects. Hygroma and pump pocket seromas may occur after pump placement but in most cases, self-resolve.
Intraventricular opioid delivery with morphine can be used for patients using an Omaya reservoir and can result in good initial pain relief. However, the evidence for this is limited and there are associated adverse effects including respiratory depression, sedation, and confusion as compared to the epidural or intrathecal route (108).
Refractory pain at the end of life
There remain a small percentage of patients who experience severe unrelenting pain at the end of life, despite appropriate pharmacologic and non-pharmacologic approaches. When this is the case sedation may be considered. At the end of life, when the overwhelming goal of care is preservation of patient comfort, the provision of adequate relief of symptoms must be pursued even in the setting of a narrow therapeutic index for the necessary palliative treatments. In this context, sedation is a medically indicated and proportionate therapeutic response to the refractory symptoms, which cannot be otherwise relieved. Appeal to the patients also underwrites the moral legitimacy of sedation in the management of otherwise intolerable pain at the end of life (109).
Conclusions
Pain is a very common symptom in individuals with cancer. It is recognized as a serious problem and for its impact on quality of life. Despite the available knowledge regarding pain management, many advances still need to take place to decrease the incidence of unrelieved pain. Performing a thorough assessment that considers physical, psychosocial, and spiritual needs, with frequent reassessment, and addressing clinician and patient barriers can provide a solid foundation toward successful pain management. We, as health professionals, may not always be able to cure the cancer or totally relieve the pain; however, we can help patients find meaning in their pain. The goal is to heal by helping the patient find a sense of wholeness in life.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Portenoy RK, Kornblith AB, Wong G, et al. Pain in ovarian cancer patients. Prevalence, characteristics, and associated symptoms. Cancer 1994;74:907-15. [PubMed]
- Beck SL, Falkson G. Prevalence and management of cancer pain in South Africa. Pain 2001;94:75-84. [PubMed]
- Chang VT, Hwang SS, Feuerman M, et al. Symptom and quality of life survey of medical oncology patients at a veterans affairs medical center: a role for symptom assessment. Cancer 2000;88:1175-83. [PubMed]
- Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer 1982;50:1913-8. [PubMed]
- Dorrepaal KL, Aaronson NK, van Dam FS. Pain experience and pain management among hospitalized cancer patients. A clinical study. Cancer 1989;63:593-8. [PubMed]
- Ger LP, Ho ST, Wang JJ, et al. The prevalence and severity of cancer pain: a study of newly-diagnosed cancer patients in Taiwan. J Pain Symptom Manage 1998;15:285-93. [PubMed]
- Greenwald HP, Bonica JJ, Bergner M. The prevalence of pain in four cancers. Cancer 1987;60:2563-9. [PubMed]
- Lin CC, Lai YL, Ward SE. Effect of cancer pain on performance status, mood states, and level of hope among Taiwanese cancer patients. J Pain Symptom Manage 2003;25:29-37. [PubMed]
- Menzies K, Murray J, Wilcock A. Audit of cancer pain management in a cancer centre. Int J Palliat Nurs 2000;6:443-7. [PubMed]
- Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res 1994;3:183-9. [PubMed]
- Ripamonti C, Zecca E, Brunelli C, et al. Pain experienced by patients hospitalized at the National Cancer Institute of Milan: research project “towards a pain-free hospital”. Tumori 2000;86:412-8. [PubMed]
- Rustøen T, Fossa SD, Skarstein J, et al. The impact of demographic and disease-specific variables on pain in cancer patients. J Pain Symptom Manage 2003;26:696-704. [PubMed]
- Sandblom G, Carlsson P, Sigsjo P, et al. Pain and health-related quality of life in a geographically defined population of men with prostate cancer. Br J Cancer 2001;85:497-503. [PubMed]
- Strohbuecker B, Mayer H, Evers GC, et al. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage 2005;29:498-506. [PubMed]
- Wells N. Pain intensity and pain interference in hospitalized patients with cancer. Oncol Nurs Forum 2000;27:985-91. [PubMed]
- Wang XS, Cleeland CS, Mendoza TR, et al. The effects of pain severity on health-related quality of life: a study of Chinese cancer patients. Cancer 1999;86:1848-55. [PubMed]
- Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck 1999;21:531-7. [PubMed]
- Harrison LB, Zelefsky MJ, Pfister DG, et al. Detailed quality of life assessment in patients treated with primary radiotherapy for squamous cell cancer of the base of the tongue. Head Neck 1997;19:169-75. [PubMed]
- Henningsohn L, Wijkstrom H, Dickman PW, et al. Distressful symptoms after radical cystectomy with urinary diversion for urinary bladder cancer: a Swedish population-based study. Eur Urol 2001;40:151-62. [PubMed]
- Rietman JS, Dijkstra PU, Debreczeni R, et al. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil 2004;26:78-84. [PubMed]
- Hwang SS, Chang VT, Cogswell J, et al. Study of unmet needs in symptomatic veterans with advanced cancer: incidence, independent predictors and unmet needs outcome model. J Pain Symptom Manage 2004;28:421-32. [PubMed]
- Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65-76. [PubMed]
- Azevedo São Leão Ferreira K, Kimura M, Jacobsen Teixeira M. The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer 2006;14:1086-93. [PubMed]
- Miguel R. Interventional treatment of cancer pain: the fourth step in the World Health Organization analgesic ladder? Cancer Control 2000;7:149-56. [PubMed]
- Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management. Stepping up the quality of its evaluation. JAMA 1995;274:1870-3. [PubMed]
- Eisenberg E MF, Birkhahm J, Paladin A, et al. Time to modify the WHO analgesic ladder? Pain Clin Update 2005;8:1-4.
- McNicol E, Strassels S, Goudas L, et al. Nonsteroidal anti-inflammatory drugs, alone or combined with opioids, for cancer pain: a systematic review. J Clin Oncol 2004;22:1975-92. [PubMed]
- Torres LM, Pernia A, Martinez-Vazquez J. From the stairs to the escalator. Rev Soc Esp Dolor 2002;9:289-90.
- Twycross R, Harcourt J, Bergl S. A survey of pain in patients with advanced cancer. J Pain Symptom Manage 1996;12:273-82. [PubMed]
- Brescia FJ, Portenoy RK, Ryan M, et al. Pain, opioid use, and survival in hospitalized patients with advanced cancer. J Clin Oncol 1992;10:149-55. [PubMed]
- Ahles TA, Ruckdeschel JC, Blanchard EB. Cancer-related pain--I. Prevalence in an outpatient setting as a function of stage of disease and type of cancer. J Psychosom Res 1984;28:115-9. [PubMed]
- Grond S, Zech D, Diefenbach C, et al. Assessment of cancer pain: a prospective evaluation in 2266 cancer patients referred to a pain service. Pain 1996;64:107-14. [PubMed]
- Turk DC, Okifuji A. Assessment of patients’ reporting of pain: an integrated perspective. Lancet 1999;353:1784-8. [PubMed]
- Weiner DK, Hanlon JT. Pain in nursing home residents: management strategies. Drugs Aging 2001;18:13-29. [PubMed]
- Weiner D, Peterson B, Keefe F. Evaluating persistent pain in long term care residents: what role for pain maps? Pain 1998;76:249-57. [PubMed]
- Herr KA, Mobily PR, Kohout FJ, et al. Evaluation of the Faces Pain Scale for use with the elderly. Clin J Pain 1998;14:29-38. [PubMed]
- Ventafridda V, Tamburini M, Caraceni A, et al. A validation study of the WHO method for cancer pain relief. Cancer 1987;59:850-6. [PubMed]
- Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012;13:e58-68. [PubMed]
- Culpepper-Morgan JA, Inturrisi CE, Portenoy RK, et al. Treatment of opioid-induced constipation with oral naloxone: a pilot study. Clin Pharmacol Ther 1992;52:90-5. [PubMed]
- Walsh D, Rivera NI, Davis MP, et al. Strategies for pain management: cleveland clinic foundation guidelines for opioid dosing for cancer pain. Support Cancer Ther 2004;1:157-64. [PubMed]
- Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007;5:1126-39. [PubMed]
- Clemens KE, Klaschik E. Management of constipation in palliative care patients. Curr Opin Support Palliat Care 2008;2:22-7. [PubMed]
- Wong JO, Chiu GL, Tsao CJ, et al. Comparison of oral controlled-release morphine with transdermal fentanyl in terminal cancer pain. Acta Anaesthesiol Sin 1997;35:25-32. [PubMed]
- Tassinari D, Sartori S, Tamburini E, et al. Transdermal fentanyl as a front-line approach to moderate-severe pain: a meta-analysis of randomized clinical trials. J Palliat Care 2009;25:172-80. [PubMed]
- Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008;358:2332-43. [PubMed]
- Portenoy RK, Thomas J, Moehl Boatwright ML, et al. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manage 2008;35:458-68. [PubMed]
- Candy B, Jones L, Goodman ML, et al. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev 2011;(1):CD003448. [PubMed]
- Sykes NP. An investigation of the ability of oral naloxone to correct opioid-related constipation in patients with advanced cancer. Palliat Med 1996;10:135-44. [PubMed]
- Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain 2011;12:185-93. [PubMed]
- Kurita GP, Sjogren P, Ekholm O, et al. Prevalence and predictors of cognitive dysfunction in opioid-treated patients with cancer: a multinational study. J Clin Oncol 2011;29:1297-303. [PubMed]
- Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer 1995;3:135-8. [PubMed]
- Webster L, Andrews M, Stoddard G. Modafinil treatment of opioid-induced sedation. Pain Med 2003;4:135-40. [PubMed]
- Kreeger L, Duncan A, Cowap J. Psychostimulants used for opioid-induced drowsiness. J Pain Symptom Manage 1996;11:1-2. [PubMed]
- Mercadante S, Serretta R, Casuccio A. Effects of caffeine as an adjuvant to morphine in advanced cancer patients. A randomized, double-blind, placebo-controlled, crossover study. J Pain Symptom Manage 2001;21:369-72. [PubMed]
- McNicol E, Horowicz-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain 2003;4:231-56. [PubMed]
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002;10:231-6. [PubMed]
- Sussman G, Shurman J, Creed MR, et al. Intravenous ondansetron for the control of opioid-induced nausea and vomiting. International S3AA3013 Study Group. Clin Ther 1999;21:1216-27. [PubMed]
- Okamoto Y, Tsuneto S, Tanimukai H, et al. Can gradual dose titration of ketamine for management of neuropathic pain prevent psychotomimetic effects in patients with advanced cancer? Am J Hosp Palliat Care 2013;30:450-4. [PubMed]
- Cherny N, Ripamonti C, Pereira J, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol 2001;19:2542-54. [PubMed]
- McDonald PP, Clayton A. Regular subcutaneous bolus morphine via an indwelling cannula for pain from advanced cancer. Palliat Med 1991;5:323-9.
- Stone P, Minton O. European Palliative Care Research collaborative pain guidelines. Central side-effects management: what is the evidence to support best practice in the management of sedation, cognitive impairment and myoclonus? Palliat Med 2011;25:431-41. [PubMed]
- Eisele JH Jr, Grigsby EJ, Dea G. Clonazepam treatment of myoclonic contractions associated with high-dose opioids: case report. Pain 1992;49:231-2. [PubMed]
- Induru RR, Davis MP. Buprenorphine for neuropathic pain--targeting hyperalgesia. Am J Hosp Palliat Care 2009;26:470-3. [PubMed]
- Hermens JM, Ebertz JM, Hanifin JM, et al. Comparison of histamine release in human skin mast cells induced by morphine, fentanyl, and oxymorphone. Anesthesiology 1985;62:124-9. [PubMed]
- Zylicz Z, Smits C, Krajnik M. Paroxetine for pruritus in advanced cancer. J Pain Symptom Manage 1998;16:121-4. [PubMed]
- Friedman JD, Dello Buono FA. Opioid antagonists in the treatment of opioid-induced constipation and pruritus. Ann Pharmacother 2001;35:85-91. [PubMed]
- Galer BS, Rowbotham MC, Perander J, et al. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain 1999;80:533-8. [PubMed]
- Wang J, Pennefather S, Russell G. Low-dose naloxone in the treatment of urinary retention during extradural fentanyl causes excessive reversal of analgesia. Br J Anaesth 1998;80:565-6. [PubMed]
- Malinovsky JM, Lepage JY, Karam G, et al. Nalbuphine reverses urinary effects of epidural morphine: a case report. J Clin Anesth 2002;14:535-8. [PubMed]
- Mercadante SL, Berchovich M, Casuccio A, et al. A prospective randomized study of corticosteroids as adjuvant drugs to opioids in advanced cancer patients. Am J Hosp Palliat Care 2007;24:13-9. [PubMed]
- Popiela T, Lucchi R, Giongo F. Methylprednisolone as palliative therapy for female terminal cancer patients. The Methylprednisolone Female Preterminal Cancer Study Group. Eur J Cancer Clin Oncol 1989;25:1823-9. [PubMed]
- Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751-4. [PubMed]
- George R, Jeba J, Ramkumar G, et al. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev 2008;CD006716. [PubMed]
- Verdu B, Decosterd I, Buclin T, et al. Antidepressants for the treatment of chronic pain. Drugs 2008;68:2611-32. [PubMed]
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev 2007;(4):CD005454. [PubMed]
- Mercadante S, Arcuri E, Tirelli W, et al. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage 2000;20:246-52. [PubMed]
- Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010;39:167-79. [PubMed]
- Barbano RL, Herrmann DN, Hart-Gouleau S, et al. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol 2004;61:914-8. [PubMed]
- Gammaitoni AR, Galer BS, Onawola R, et al. Lidocaine patch 5% and its positive impact on pain qualities in osteoarthritis: results of a pilot 2-week, open-label study using the Neuropathic Pain Scale. Curr Med Res Opin 2004;20 Suppl 2:S13-9. [PubMed]
- Jones VM, Moore KA, Peterson DM. Capsaicin 8% topical patch (Qutenza)--a review of the evidence. J Pain Palliat Care Pharmacother 2011;25:32-41. [PubMed]
- Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013;309:1359-67. [PubMed]
- Caraceni A, Zecca E, Bonezzi C, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol 2004;22:2909-17. [PubMed]
- Mishra S, Bhatnagar S, Goyal GN, et al. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. Am J Hosp Palliat Care 2012;29:177-82. [PubMed]
- Bennett MI, Laird B, van Litsenburg C, et al. Pregabalin for the management of neuropathic pain in adults with cancer: a systematic review of the literature. Pain Med 2013;14:1681-8. [PubMed]
- Nasreddine W, Beydoun A. Oxcarbazepine in neuropathic pain. Expert Opin Investig Drugs 2007;16:1615-25. [PubMed]
- Tremont-Lukats IW, Challapalli V, McNicol ED, et al. Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth Analg 2005;101:1738-49. [PubMed]
- Jackson K, Ashby M, Howell D, et al. The effectiveness and adverse effects profile of “burst” ketamine in refractory cancer pain: The VCOG PM 1-00 study. J Palliat Care 2010;26:176-83. [PubMed]
- Lauretti GR, Lima IC, Reis MP, et al. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology 1999;90:1528-33. [PubMed]
- Yang CY, Wong CS, Chang JY, et al. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Can J Anaesth 1996;43:379-83. [PubMed]
- Salas S, Frasca M, Planchet-Barraud B, et al. Ketamine analgesic effect by continuous intravenous infusion in refractory cancer pain: considerations about the clinical research in palliative care. J Palliat Med 2012;15:287-93. [PubMed]
- Hardy J, Quinn S, Fazekas B, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol 2012;30:3611-7. [PubMed]
- Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev 2012;11:CD003351. [PubMed]
- Okamoto Y, Tsuneto S, Matsuda Y, et al. A retrospective chart review of the antiemetic effectiveness of risperidone in refractory opioid-induced nausea and vomiting in advanced cancer patients. J Pain Symptom Manage 2007;34:217-22. [PubMed]
- Cleeland CS, Body JJ, Stopeck A, et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer 2013;119:832-8. [PubMed]
- Xie J, Namjoshi M, Wu EQ, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm 2011;17:621-43. [PubMed]
- Davis MP, Hallerberg G. Palliative Medicine Study Group of the Multinational Association of Supportive Care in C. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy or radiation. J Pain Symptom Manage 2010;39:756-67. [PubMed]
- Chew C, Craig L, Edwards R, et al. Safety and efficacy of percutaneous vertebroplasty in malignancy: a systematic review. Clin Radiol 2011;66:63-72. [PubMed]
- Bouza C, Lopez-Cuadrado T, Cediel P, et al. Balloon kyphoplasty in malignant spinal fractures: a systematic review and meta-analysis. BMC Palliat Care 2009;8:12. [PubMed]
- Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12:225-35. [PubMed]
- Terpos E, Morgan G, Dimopoulos MA, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol 2013;31:2347-57. [PubMed]
- Fujii T, Nagaro T, Tsubota S, et al. Case reports: management of intractable upper extremity pain with continuous subarachnoid block at the low cervical level without impairment of upper extremity function. Anesth Analg 2010;110:1721-4. [PubMed]
- Vranken JH, Zuurmond WW, de Lange JJ. Continuous brachial plexus block as treatment for the Pancoast syndrome. Clin J Pain 2000;16:327-33. [PubMed]
- Lihua P, Su M, Zejun Z, et al. Spinal cord stimulation for cancer-related pain in adults. Cochrane Database Syst Rev 2013;2:CD009389. [PubMed]
- Ballantyne JC, Carwood CM. Comparative efficacy of epidural, subarachnoid, and intracerebroventricular opioids in patients with pain due to cancer. Cochrane Database Syst Rev 2005;(1):CD005178. [PubMed]
- van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain 1999;15:166-72. [PubMed]
- Eisenach JC, DuPen S, Dubois M, et al. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain 1995;61:391-9. [PubMed]
- Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol 2002;20:4040-9. [PubMed]
- Dennis GC, DeWitty RL. Long-term intraventricular infusion of morphine for intractable pain in cancer of the head and neck. Neurosurgery 1990;26:404-7; discussion 7-8. [PubMed]
- Cherny NI. The management of cancer pain. CA Cancer J Clin 2000;50:70-116. [PubMed]