Chemotherapy for advanced cancers
Introduction
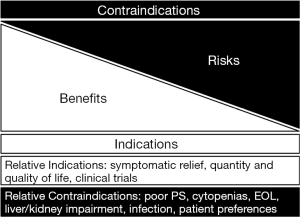
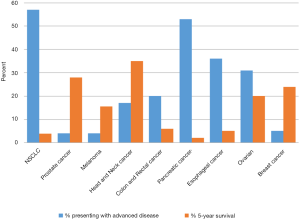
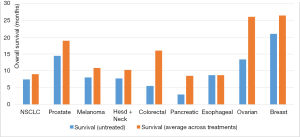
The use of palliative chemotherapy in patients with advanced cancers is complex. Palliative chemotherapy has been associated with increased risk of cardiopulmonary resuscitation and death in an intensive care unit (ICU) (1,2). However, the length of survival for patients with advanced cancer depends on the type of malignancy and its sensitivity to chemotherapy, but is generally worse than for patients without advanced cancer (Figure 1). The goals of chemotherapy for incurable cancer are prolongation of life, alleviation of symptoms, and maintenance or improvement in quality of life (QOL), despite the toxicity associated with treatment. Important factors for treatment decisions also include the patient’s preferences, comorbidities, and performance status (PS), a measure of a patient’s activity level and general well-being (Figure 2). The indications and benefits for chemotherapy (Table 1, Figure 3) need to be continually reassessed to ensure the risk-benefit ratio favors treatment (Figure 4).
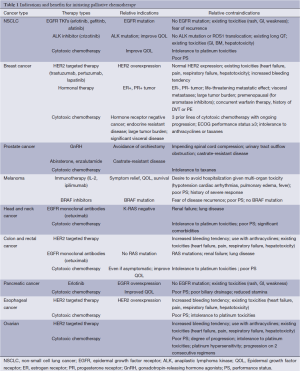
Full table
This review will discuss the decision-making process involved in the treatment of some common advanced malignancies. Each section begins by defining what constitutes “advanced” disease. The prognosis for each malignancy and the indications for treatment are explained. The chemotherapy and palliative care treatment options are discussed.
Melanoma
In 2014, there will be an estimated 76,100 new cases and 9,710 deaths from melanoma in the United States. Melanoma is the 5th most common cancer among men and the 7th among women (4).
Advanced melanoma
Four percent of patients in the U.S. present with advanced melanoma, meaning it is stage IV, metastatic, and incurable (3).
Prognosis
The probability of surviving 5 years after diagnosis with metastatic melanoma is approximately 15.5% (3). Median overall survival (OS) of untreated or progressive disease generally ranges from 4 months to 1 year depending on metastatic sites (5).
Treatment
Immunotherapy
Interleukin-2 (IL-2) is a T-cell growth factor that shows efficacy in 15-20% of patients and a complete response (CR) in 4-6% of patients. Most importantly, 80-90% of responders remain alive after 10 to 15 years (6-8). IL-2 can cause hypotension, cardiac arrhythmias, pulmonary edema and fever. It requires significant supportive care (Table 2) (9).
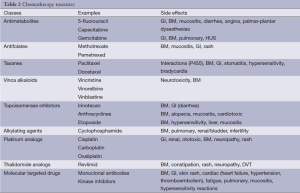
Full table
Ipilimumab, a monoclonal antibody that targets the inhibitive CTLA-4 receptor on T cells, has shown a 20% response rate (RR) to therapy with an OS increase of 2 to 4 months (10,11). Twenty to 25 percent of patients survive beyond 3 years. Immunotherapies may cause transient worsening of disease or have long latent periods before response (12).
Targeted therapy
Approximately 40-60% of melanomas have a BRAF gene mutation. The most common BRAF mutation in melanoma leads to an amino-acid substitution (V600) that upregulates the MAPK signaling pathway (13). Vemurafenib and dabrafenib are BRAF inhibitors. Among patients with a V600 mutation, vemurafenib and dabrafenib each had a 50% RR and demonstrated extended progression-free survival (PFS) and OS by approximately 3 to 6 months (14-16). Patients responsive to BRAF inhibitors almost always have disease recurrence (17).
Trametinib is a MEK1/2 inhibitor that has been shown to have a 22% RR and improve PFS by approximately 3 months (18). Combination with dabrafenib had RRs of 76% and nearly doubled PFS seen with dabrafenib alone (19).
At this time, there is no data on the superiority or order of administration between immunotherapy and BRAF inhibition. Guidelines recommend initial treatment of advanced melanoma based on mutation status and PS (20,21). Patients with good PS (regardless of mutation) can be treated with immunotherapy as first-line, and BRAF+ patients can subsequently be treated with targeted therapy. BRAF+ patients with poor PS or brain metastases should be started on targeted therapy before ipilimumab. Ipilimumab is recommended for BRAF- patients with poor PS.
Cytotoxic chemotherapy
No single or combination chemotherapy agents have been shown to improve OS in clinical trials (22). Dacarbazine, the only FDA-approved chemotherapeutic agent, has an overall RR (mostly partial) of 10-25% and durations of survival from 5.6 to 11 months (23,24).
Role of palliative care
Palliative care for patients with advanced melanoma involves the management of a multitude of symptoms, depending on metastatic site(s). Fatigue, pain, and nausea are common. Localized therapy with surgery or radiation has a role in palliation of advanced melanoma for symptomatic relief (21). Palliative resection of gastrointestinal metastases can relieve symptoms (abdominal pain, constipation, bowel obstruction, anorexia, nausea, vomiting, bleeding) for patients with melanoma found in the bowel (25). About 20-50% of patients with advanced melanoma present with brain metastases and associated symptoms, including headaches, mental status changes, seizures and bleeds (26). Surgical resection and radiation are options for patients with metastatic brain lesions (27).
Non-small cell lung cancer (NSCLC)
In 2014, there will be an estimated 224,210 new cases and 159,260 deaths from lung cancer in the United States. Lung cancer represents the second most common cancer diagnosis and the leading cause of death for both men and women. NSCLC represents over 80-85% of this disease (4).
Advanced NSCLC
Fifty-seven percent of patients present with stage IV disease (3). Patients with distant, disseminated metastases that cannot be cured through localized radiation or surgical therapy, or patients with relapsed disease, have advanced NSCLC.
Prognosis
The 5-year survival rate with advanced NSCLC is 3.9% (3). Median survival rates of advanced NSCLC depend on location of metastases: extrathoracic metastases have a median survival of 4 to 7 months, pleural disease 7 to 10 months, and contralateral lung 9 to 11 months (28). Treatment at this stage can offer a median increase in survival of 1.5 months without detracting significantly from QOL (29).
Treatment
Targeted therapy
Mutation-targeted therapies have proven efficacious in NSCLC. Treatment of advanced NSCLC depends on genetic markers and PS of patients. Patients with ECOG PS 3-4 [approximately 35% of patients (30)] have only been shown to benefit from targeted, and not cytotoxic, therapy (31-33).
Epidermal growth factor receptor [(EGFR), HER-1/ErbB1] is a cell-surface receptor which, when overexpressed, results in inappropriate stimulation of oncogene pathways (34). Ten to 15 percent of NSCLC adenocarcinomas in the U.S. have mutations in EGFR, which are found more frequently among nonsmokers, women, and in the Asian population (35). Among patients with EGFR mutations, treatment with erlotinib (36,37), gefitinib (38-40), or afatinib (41) can obtain RRs of approximately 50-80% and has been shown to significantly prolong PFS up to 6 months relative to chemotherapy, but without clear OS benefit (42,43). Almost all patients with EGFR treatment will acquire resistance (44).
The anaplastic lymphoma kinase (ALK) gene participates in a chromosome 2 translocation to produce multiple fusion oncogenes (45). Two to 7 percent of NSCLC adenocarcinomas in the U.S. contain ALK gene arrangements, which usually occur among younger patients with minimal smoking history (46). Crizotinib, a small molecule tyrosine kinase inhibitor, strongly inhibits ALK among other proteins. Crizotinib has shown RRs of 50-65% and improved PFS of 4 to 10 months, although the effect on OS is unclear (47-49). Notably, patients report increased QOL on crizotinib (47).
ROS1 is an insulin receptor tyrosine kinase with homology and fusion activity similar to ALK. One to 2 percent of NSCLC patients have ROS1 rearrangements. Treatment with crizotinib for patients with the ROS1 translocation is recommended whenever possible (50,51).
Bevacizumab is a monoclonal antibody that inhibits vascular endothelial growth factor (VEGF). It is FDA-approved to treat advanced non-squamous NSCLC in combination with platinum doublets. Bevacizumab increases the RR of platinum-based regimens to 30-35% and offers a PFS and OS increase of up to 2 months (52-54).
Cytotoxic chemotherapy
Platinum-doublet chemotherapy is the preferred initial cytotoxic regimen (33). These doublets tend to increase the RR of single agents to 25-35%, PFS to 4 to 6 months, 1-year survival to 30-40%, while improving symptoms and QOL (33,55,56). Single-agent treatment may be appropriate for older patients or those with borderline PS (57).
Both carboplatin and cisplatin may be used in advanced NSCLC patients. Multiple meta-analyses suggest both better RR and OS for cisplatin, but carboplatin has less severe toxicity and improved QOL (58-61). Docetaxel alone not only improves OS by approximately 3 months (62), but also improves QOL compared to best supportive care (63,64). Compared to other platinum-based doublets, platinum with pemetrexed improves OS (for non-squamous patients) by approximately 2 months (65,66). Pemetrexed also has less toxicity compared to docetaxel (67). Paclitaxel, gemcitabine, and vinorelbine are also used (68).
Role of palliative care
Palliative care for patients with advanced NSCLC includes focusing on dyspnea, fatigue, pain and depression (69). Opioids help with dyspnea in NSCLC (69). Brain and bone metastases are common in these patients (70,71), warranting palliative relief (72). Early palliative care consultation has been shown not only to improve QOL, but also to extend survival by a median of 3 months (73).
Breast cancer
In 2014, there will be an estimated 235,030 new cases and 40,430 deaths from breast cancer in the United States. Among U.S. women, breast cancer is the most common cancer and the second leading cause of cancer death (4).
Advanced breast cancer
Five percent of patients with breast cancer present with metastatic disease (3). Patients with advanced breast cancer have disease that cannot be removed with surgery or radiation alone.
Prognosis
The 5-year survival for advanced breast cancer is 24% (3). The median OS of the disease is 18 to 24 months (74). Median OS can increase by 3 to 5 months with chemotherapy and 4 to 8 months with endocrine and targeted therapies (75).
Treatment
Hormonal therapy
About 60% of breast cancers are considered hormone-receptor positive through expression of estrogen receptor (ER) and/or progesterone receptor (PR) (76). Tumors expressing both have a 70% likelihood of response to hormone therapy, while tumors expressing only one have a 40% likelihood, and tumors expressing neither have a 10% likelihood (76,77). A Cochrane meta-analysis recommended initial management with endocrine therapy for hormone-positive patients over chemotherapy unless patients had life-threatening, rapid or extensive visceral metastases (78). Selective estrogen receptor modulators (SERMs) such as tamoxifen are estrogen antagonists in the breast and agonists in the bone and uterus. While premenopausal women benefit from tamoxifen (24% RR), it demonstrates the greatest efficacy among postmenopausal women with both ER+ and PR+ disease (86% RR) (79,80). The median OS for SERMs among advanced breast cancer patients is 27.7 months, with median time to progression of 6 months (81). Aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane block aromatase from synthesizing peripheral estrogens. They are contraindicated in premenopausal women as they can induce ovarian estrogen upsurge. Among postmenopausal women, AIs show a significant OS benefit over SERMs/other endocrine therapies by approximately 3 months as well as decreased risk of vaginal bleeding and thromboembolic events (82-85). Fulvestrant, an ER antagonist, shows similar efficacy to AIs in women with breast cancer progression on other antiestrogens (86-88). Use of the combination of fulvestrant with AIs, or SERMs remains controversial (89-91).
Cytotoxic chemotherapy
Chemotherapy results in higher and more rapid RRs than endocrine therapy, and is often the initial treatment strategy for highly symptomatic metastatic breast cancer patients even if hormone-positive, especially those with visceral metastases. Numerous agents have demonstrated efficacy with median OS 11 to 15 months, PFS 3 to 7 months, RR 85%. Anthracyclines and taxanes are the most commonly used and can be given as infrequently as once every three weeks with preserved efficacy (92-94). Sequential single-agent chemotherapy with agents of different classes shows increased PFS, similar OS and lower toxicity, but slower onset of effect compared to combination chemotherapy (95). NCCN guidelines suggest transition to supportive care only after 3 sequential lines of chemotherapy with progression or ECOG PS ≥3 (96).
Targeted therapy
Fifteen to 20 percent of patients with breast cancer demonstrate HER2 overexpression (97). A meta-analysis of eight trials concluded that addition of any HER2-targeted agent to an existing chemotherapeutic regimen for a patient with susceptible disease improves OS, PFS and RR (98). Trastuzumab, a monoclonal antibody to the HER2/Neu extracellular receptor, added to a standard chemotherapeutic regimen demonstrates an increase in OS of 2 to 5 months (25.1 vs. 20.3 months), PFS of 3 months (7.4 vs. 4.6 months), and RR of 18% (99,100). T-DM1, a conjugate of transtuzumab with an antimicrotubule agent, increases median PFS by 4 to 5 months and OS by 6 months, and RR by 13% among women who progress on trastuzumab (101,102). Pertuzumab is a monoclonal antibody directed against HER2 dimerization. In the CLEOPATRA study, the addition of pertuzumab increased PFS from 12.5 to 18.7 months compared to traztuzumab and a taxane alone (103,104). Lapatinib, a tyrosine kinase inhibitor against EGFR1 and HER2, has demonstrated inferior outcomes to trastuzumab in preliminary studies (105).
Role of palliative care
Palliative care for patients with advanced breast cancer can aid in the management of bone or visceral pain, cognitive impairment, depression and lymphedema (106). Osteoclast inhibitors such as denosumab, a RANK ligand antibody, and bisphosphonates reduce the risk of skeletal events and reduce bone pain (107,108). Osteonecrosis of the jaw can be reduced through a preventative dentistry exam prior to osteoclast inhibitor treatment (109). Psychological interventions (cognitive behavioral therapy, supportive-expressive therapy, etc.) have shown to be beneficial (110).
Prostate cancer
In 2014, there will be an estimated 233,000 new cases of prostate cancer in the United States (4). There will be 29,480 estimated deaths due to prostate cancer in 2014 (4). Prostate cancer is the most common cancer in men (4).
Advanced prostate cancer
Patients with metastatic or disseminated disease are considered to have advanced prostate cancer. Approximately 4% of patients in the U.S. present with distant disease (3).
Prognosis
The 5-year survival for advanced prostate cancer patients is 28% (3). The sites of metastatic disease influence OS. A phase III trial comparing docetaxel plus prednisone to mitoxantrone plus prednisone showed decreasing OS as the extent of disease increased from limited to lymph nodes only (median 27 months), to bones only (19 months), to lung with or without bone and lymph nodes (14 months), and to liver regardless of other sites of disease (10 months) (111).
Treatment
Androgen deprivation therapy (ADT)
Systemic treatment of metastatic prostate cancer usually involves ADT to help lower serum testosterone levels to castrate levels. The optimal time to initiate systemic therapy is uncertain for patients with metastatic disease, but several trials have found that immediate compared to delayed ADT was associated with a statistically significant decrease in prostate cancer-related death although there was no OS benefit (112). A trial seeking to assess whether intermittent ADT was non-inferior to continuous therapy found a median survival of 5.8 years in the continuous-therapy group and 5.1 years in the intermittent-therapy group (113). Gonadotropin releasing hormone agonists (GnRH) are used as “medical castration”. A meta-analysis comparing a GnRH agonist with orchiectomy found no significant difference between the two groups (median survivals of 20 to 40 months) (114). GnRH agonists can cause a transient surge of luteinizing hormone known as the “flare phenomenon” which can initially worsen disease. This proves problematic in cases such as impending epidural spinal cord compression or urinary tract outflow obstruction. Antiandrogens (flutamide, bicalutamide) may be useful in preventing this phenomenon.
Castrate resistant disease
Patients with disease progression (PSA increase, new metastases, progression of metastases) while receiving ADT are considered to have castrate resistant disease. Abiraterone and enzalutamide have both shown significant improvements in OS compared with placebo in phase III trials with castrate resistant prostate cancer. Abiraterone, an oral small molecule that blocks the synthesis of androgens, is approved for patients who have metastatic castrate resistant prostate cancer based on phase III data. Abiraterone prolonged OS compared with prednisone alone (14.8 vs. 10.9 months) in men who had previously been treated with docetaxel and in those who were chemotherapy naïve (115,116). The phase III data for enzalutamide in metastatic, castration resistant patients showed a median OS of 18.4 months versus 13.6 months for placebo (117). Alternative endocrine therapies such as glucocorticoids, ketoconazole (118), and antiandrogens (bicalutamide, flutamide) (119) may help stabilize disease but have not shown OS benefits.
Sipuleucel-T, an autologous dendritic cell therapeutic vaccine designed to enhance the immune T cell response to prostatic acid phosphatase, is a treatment option in asymptomatic or minimally symptomatic men (PS 0 or 1) with metastatic, castration resistant prostate cancer. Phase III data for sipuleucel-T showed improvement in OS (25.8 vs. 21.7 months in the placebo group) (120).
Another option for advanced prostate cancer and bone metastases is radium-223. A phase III trial randomly assigned patients to receive radium or placebo. This was trial was terminated for efficacy, as it showed a survival benefit (14.9 vs. 11.3 months) (121).
Cytotoxic chemotherapy
Taxanes are the standard chemotherapy agents for castrate-resistant prostate cancer. Their use is supported by phase III data showing docetaxel in combination with prednisone significantly prolonged OS compared to mitoxantrone plus prednisone (19.2 vs. 16.3 months) (122).
Role of palliative care
Palliative care for patients with advanced prostate cancer includes management of bone metastases, bone pain, urinary tract symptoms, pelvic pain, hematuria, and obstructive rectal symptoms. Bone metastases in prostate cancer are usually osteoblastic lesions to the axial skeleton that cause pain, functional impairment, and debility. Radiotherapy (123), osteoclast inhibition (bisphosphonates, denosumab) (124), radium-223, and adequate analgesia are options for treating painful bone metastases. Radiation can help relieve the following: rectal symptoms (75%), pelvic pain (69%), urinary obstruction (54%) and haematuria (42%) (125).
Head and neck (H&N) cancer
H&N cancer includes the oral cavity, pharynx, larynx, nasal cavity, paranasal sinuses, thyroid, and salivary glands. In 2014, there will be over 100,000 estimated new cases of H&N cancer in the United States: 42,440 oropharynx, 12,630 larynx, and 62,980 thyroid (4). There will be over 13,000 deaths in the U.S. attributed to H&N cancer: 8,390 oropharynx, 3,610 larynx, and 1,890 thyroid (4).
Advanced H&N cancer
The focus of this discussion will be patients with recurrent or metastatic H&N squamous cell cancer (SCC). Approximately 17-18% of patients in the U.S. present with advanced oropharyngeal or laryngeal cancer (3).
Prognosis
The 5-year survival for advanced oropharyngeal and laryngeal cancer patients is 17-18% (3). The median OS for metastatic H&N carcinoma treated with cisplatin-based combination chemotherapy has been shown to be 7.8 months. Weight loss, poor PS, and prior RT were found to predict worse outcomes (126). The HPV status of H&N cancer plays an important role in prognostication, as HPV positive tumors have a better prognosis than cancers not associated with HPV. HPV positive tumors may have lower incidence of metastases (10% vs. 15%), but data suggests that HPV positive cancers develop metastases later and in different patterns than HPV negative cancers (127).
Treatment
Cytotoxic chemotherapy
The backbone of chemotherapy for H&N SCC is usually platinum compounds (cisplatin, carboplatin). Carboplatin is associated with less neurotoxicity, nephrotoxicity, and nausea and vomiting compared with cisplatin. Data suggests these agents result in similar responses (128). Other common agents include: taxanes (docetaxel, paclitaxel), methotrexate, 5-fluorouracil (5-FU), and cetuximab. Combination chemotherapy is generally preferred, but for patients with poor PS or significant comorbidities, single agent therapy with carboplatin, paclitaxel, or cetuximab can be attempted. For patients with good PS and favorable prognostic features, combination chemotherapy using platinum plus fluorouracil, with or without cetuximab, or platinum plus a taxane, is usually employed. A phase III trial comparing cisplatin plus fluorouracil to cisplatin plus paclitaxel showed no significant difference in median survival (8.7 vs. 8.1 months) (129).
Second-line chemotherapy must take into consideration the patient’s prior treatments, overall condition, and the toxicity profiles of future chemotherapy. Methotrexate, docetaxel, cetuximab, and gemcitabine have shown activity in previously treated patients (130-132).
Targeted therapy
Cetuximab, a monoclonal antibody to the EGFR, has demonstrated activity in H&N SCC. The EXTREME trial studied cetuximab plus platinum-based chemotherapy as first-line treatment in patients with recurrent or metastatic H&N SCC (128). The addition of cetuximab prolonged median OS from 7.4 months in the chemotherapy-alone group to 10.1 months in the group that received chemotherapy plus cetuximab (128).
Panitumumab, another monoclonal antibody targeting the EGFR receptor, has activity in advanced H&N SCC. The SPECTRUM phase III trial of cisplatin plus 5-FU, with or without panitumumab, showed a non-significant improvement in OS (11.1 vs. 9.0 months in the control group) (133).
Role of palliative care
The radiation, surgery, and chemotherapy that many H&N patients receive can cause multiple side effects, including: xerostomia, thyroid disorders (134), dysphagia and weight loss which may prompt the use of feeding tubes. Feeding tubes may help prevent cachexia and improve QOL (135), but the multitude of adverse effects related to feeding tubes merits the frequent reassessment of their use (136). Speech pathologists and nutrition specialists can play a valuable role as part of the multidisciplinary treatment team. Patients with H&N may SCC struggle with mood disorders (137), and studies have shown benefits with antidepressant use in H&N cancer (138).
Colorectal cancer (CRC)
In 2014, there will be an estimated 136,830 new cases and 50,310 deaths from CRC in the United States. CRC represents the third most common cancer and the third leading cause of cancer deaths among both men and women (4).
Advanced CRC
Twenty percent of patients with CRC present with metastatic disease (3). Patients with advanced CRC demonstrate metastatic disease that cannot be surgically removed or cured.
Prognosis
Six percent of advanced colon cancer patients live to 5 years (3). The median OS with best supportive care alone is approximately 5 to 6 months (139). Chemotherapy can prolong PFS and OS by approximately 3 to 18 months (139,140).
Treatment
Cytotoxic chemotherapy
Palliative chemotherapy should be initiated at diagnosis even if patients are asymptomatic. Length of OS correlates strongly with exposure to all of the active chemotherapeutic agents (5-FU, irinotecan and oxaliplatin), rather than specific sequences of chemotherapy (141-143). Techniques such as chemotherapy switching, drug holidays, and maintenance therapy are frequently employed (144-146). Patients with poor PS can gain benefit from chemotherapy if toxicities permit (147).
Chemotherapy commonly used in CRC includes: oxaliplatin with fluorouracil/leucovorin (FOLFOX) or capecitabine (CapeOX), irinotecan with fluorouracil/leucovorin (FOLFIRI) and chemotherapy plus targeted therapies (148). FOLFOX and FOLFIRI can be combined with all of the targeted chemotherapies described below, albeit in very specific regimens, as well as with each other. Patients prefer the convenience of oral capecitabine, although outpatient fluorouracil/leucovorin has been associated with better QOL (149). Irinotecan as an independent agent has demonstrated an OS increase of 3 months (to 9 months) (150). FOLFIRI can improve PFS of 6 to 8 months and OS of 15 to 20 months (151-154). In studies of second-line therapy, FOLFIRI and irinotecan are comparable regarding PFS (3 to 6 months), toxicities, and QOL (155,156).
Oxaliplatin alone shows weak efficacy in advanced CRC, with a PFS of 4 months (157). FOLFOX demonstrates a median PFS of 6 to 9 months and OS of 11 to 20 months (158,159). Capecitabine and oxaliplatin (CapeOX or XELOX) demonstrate a median PFS of 8 months and OS of 20 months; this regimen is considered non-inferior to FOLFOX (160-162). Peripheral neuropathy from oxaliplatin can be alleviated through intermittent dosing or stopping the regimen after 3 months (163,164). FOLFOX and FOLFIRI have similar efficacies (165,166). The combination of oxaliplatin, irinotecan and fluorouracil/leucovorin (FOLFOXIRI) offers a slightly improved median OS of 21.5 to 22.6 months and PFS of 8.4 to 9.8 months. This regimen has greater toxicity than either FOLFOX or FOLFIRI (167,168).
Targeted therapy
Bevacizumab can be used as an adjunct for CRC (148). It adds approximately 2 months to PFS and OS but with increased toxicities (169-174). Aflibercept inhibits VEGF by acting as a “decoy receptor” and binding to circulating VEGF-A, -B or placental growth factor (175). It is FDA-approved as an adjunct with FOLFIRI or irinotecan for patients previously treated with oxaliplatin-based regimens (148).
ASCO recommends testing patients for RAS mutations prior to considering agents targeting EGFR (cetuximab or panitumumab), as mutations in the RAS pathway override the effects of these drugs (176). For wild-type RAS patients, cetuximab improves QOL and doubles the success of supportive care alone with a median PFS of 3.7 months and OS of 9.5 months (177-179). Panitumumab demonstrates similar efficacy to cetuximab (180). Regorafenib, an oral multikinase inhibitor, demonstrates inhibition of VEGF, PDGF, FGFR1, KIT, RET and B-RAF (181). It has been FDA-approved in metastatic CRC patients (148). A phase III trial of patients with progression through multiple standard therapies demonstrated an increase of 1.4 months in OS (182). Multiple toxicities including hand-foot-skin reaction, rash, diarrhea, fatigue, neutropenia, and fatal hepatic toxicity were observed in 1.4% of patients (183).
Role of palliative care
Palliative care for patients with advanced CRC often involves treating pain, nausea, vomiting, and anorexia (184,185). Fifteen to 20 percent of metastatic colon cancer patients develop bowel obstruction (186). Palliative surgical options include resection and primary anastomosis, colostomy, and bypass (187). Self-expanding metal stents are a common nonsurgical alternative that have been extensively researched. There does not appear to be any significant difference in mortality between these options (188-191). Stents have the benefit of more rapid recovery and reduced likelihood of ICU or stoma requirements (192-194).
Pancreatic cancer
In 2014, there will be an estimated 46,420 new cases and 39,590 deaths from pancreatic cancer in the United States (4). Pancreatic cancer represents the fourth most common cause of cancer death among both men and women.
Advanced pancreatic cancer
Fifty-three percent of pancreatic cancer patients present with advanced disease: stage IV, with metastases to distant lymph nodes or organs, and surgical excision is not possible (3).
Prognosis
Two percent of patients with stage IV disease will survive to 5 years (3). The median survival of untreated patients with metastatic disease is measured in months. Chemotherapy can extend OS by 3 to 8 months and can improve QOL through symptom control (195).
Treatment
Cytotoxic chemotherapy
There is minimal benefit for advanced pancreatic cancer patients with poor PS, thus PS should be considered prior to initiation of chemotherapy (196,197). Chemotherapy is reasonable for patients with good PS, adequate biliary drainage, and for those able to tolerate intensive therapy. FOLFIRINOX, a combination of leucovorin, 5-FU, irinotecan and oxaliplatin, is commonly used for treating metastatic pancreatic cancer. RRs of 31.6% have been seen, along with PFS of 6.4 months, and median OS of 11.1 months (198). FOLFIRINOX has been associated with reduced deterioration in QOL compared to gemcitabine (198,199).
As monotherapy, gemcitabine has superior efficacy for advanced pancreatic cancer compared to weekly 5-FU (200). Gemcitabine can achieve RRs of 7-12%, PFS of 4 months, and median OS of 6 to 8 months along with symptom improvement (198,200). Some combination therapies with gemcitabine demonstrate improved OS, PFS and RR (201,202). Albumin-bound paclitaxel results in an increase in PFS and OS by 2 months; it has been approved by the FDA for first-line therapy in advanced pancreatic cancer patients (203). Drugs such as capecitabine, 5-FU, oxaliplatin, cisplatin, docetaxel or irinotecan also have activity in pancreatic cancer (204-218).
Second-line treatments in pancreatic cancer can improve median OS by as much as 4 to 6 months; platinum in combination with gemcitabine or 5-FU are commonly used in second-line (219). The phase III CONKO trial provided data for oxaliplatin, folinic acid and 5-FU as second line therapy after progression on gemcitabine (220). This led to the guideline recommendation of using 5-FU based therapy after progression despite gemcitabine and vice versa (221).
Targeted chemotherapy
EGFR is overexpressed in approximately 43% of pancreatic adenocarcinomas and is associated with increased tumor aggressiveness (222). The combination of erlotinib and gemcitabine is FDA approved for first-line treatment of advanced pancreatic cancer on the basis of a phase III trial showing an increase in median OS of 1 week and 5% increased 1-year survival relative to gemcitabine alone (223).
Role of palliative care
Pancreatic cancer patients may require treatment of biliary and gastric outlet obstruction, pain, depression, cachexia and malnutrition (221,224,225). Biliary obstruction occurs in up to 80% of patients with pancreatic head involvement and can cause pruritus, jaundice and anorexia (226). Stents or percutaneous biliary drainage may be necessary to alleviate patients’ symptoms (227-231). Malignant gastric outlet obstruction occurs in 15% to 25% of patients and can be managed with either endoscopic stenting or feeding tubes (232-234). Cachexia, anorexia, and weight loss occur in up to 80% of pancreatic cancer patients (235,236). Pancreatic insufficiency can contribute to cachexia, malnutrition and steatorrhea (237,238). Microencapsulated lipase supplements can alleviate these symptoms (239,240). For pain management, celiac plexus neurolysis can help (241,242). Psychological distress can be especially potent in this population (243). One study noted male advanced pancreatic cancer patients had an 11-fold increased risk of suicide (244). Patients should be monitored for symptoms of anxiety, depression and sleep disturbance throughout the disease process (245). Patients with advanced pancreatic cancer are at a 4- to 7-times increased risk of venous thromboembolism (VTE) compared to other cancers (246). Low-molecular-weight heparin is the preferred agent to prevent VTE compared to warfarin (247-250).
Esophageal cancer
In 2014, there will be an estimated 18,170 new cases and 15,450 deaths from esophageal cancer in the United States (4).
Advanced esophageal cancer
Stage IV adenocarcinoma and squamous cell carcinoma are managed similarly. Fifty to 60 percent of patients present with advanced esophageal cancer; their disease has metastasized or is deemed unresectable (3).
Prognosis
The 5-year survival of patients with advanced disease is 3-5% (3). ECOG PS ≥2, liver or peritoneal metastases are adverse prognostic factors (251). Patients without adverse factors have a median survival of 12 months, while patients with all of them have a median survival of 4 to 7 months (251). Currently, there is no clear evidence of survival benefit with chemotherapy alone (252,253). The goal of chemotherapy is to improve QOL, PS, and symptoms (252,254,255).
Treatment
Cytotoxic chemotherapy
PS should be assessed prior to administration of chemotherapy. Patients with poor PS (Karnofsky <60 or ECOG ≥2) should be managed with supportive care (256-258). No single chemotherapy regimen for esophageal cancer has demonstrated clear superiority (252). Nearly all regimens result in OS of 9 to 11 months, PFS of 6 to 7 months, with RRs of 35-48% (259,260). The most common regimens include oxaliplatin, cisplatin, 5-FU, capecitabine and/or an anthracycline (259,261,262). Irinotecan has also been investigated in this population (263). Capecitabine and oxaliplatin are considered to be as effective as 5-FU and cisplatin based on the REAL-2 trial (259). While no data exists on how many courses of therapy to pursue, NCCN guidelines suggest second-line therapy with irinotecan (264), paclitaxel (265,266) and docetaxel given their single-agent efficacy (258,267).
Targeted therapy
Approximately 7-22% of esophagogastric cancers overexpress the type II EGFR HER2 (268). The addition of trastuzumab to chemotherapy leads to a 10% increased RR and improves OS for those patients by about 3 months (269).
Role of palliative care
Palliative care for patients with advanced esophageal cancer includes treatment of dysphagia, pain, bleeding, nausea, vomiting and malnutrition (258,270). Treatment of dysphagia usually involves treatment of obstruction; options include a self-expanding metal stent, intraluminal brachytherapy, or external beam radiotherapy (271-273). Dilation or chemotherapy alone, and surgical bypass are not recommended due to their relatively low ratio of benefits to complications (272). Feeding tubes are recommended as necessary to facilitate nutritional support and ease nausea/vomiting (274,275). Esophageal cancers commonly bleed, which can be controlled with electrocoagulation, endoscopic intervention, or radiotherapy (258,276).
Ovarian
In 2014, there will be an estimated 21,980 new cases and 14,270 deaths from ovarian cancer in the United States (4). Ovarian cancer represents the fifth most deadly cancer among U.S. women, and the most common cause of gynecological cancer death.
Advanced ovarian cancer
Thirty-one percent of patients present with stage IV disease (3). Patients with distant metastases that have progressed or recurred after debulking and initial adjuvant chemotherapy with platinum are considered to have advanced disease.
Prognosis
Eighteen to 20 percent of patients with stage IV disease will survive 5 years (4,277). Historically, ovarian cancer showed PFS of 7.1 months and OS of 13.4 months with incomplete treatment (278). This has nearly doubled in the last decade (279). Current median OS depends on presence of residual tumor after debulking (54.6 months for no residual disease versus 23.9 for greater than 1 cm residual disease), extent of metastases (20.2 months for multi-site disease vs. 26.8 months for single-site disease), and PS (19.3 months for ECOG 2 vs. 32.8 months for ECOG 0) (279,280). Higher QOL is also associated with improved survival (280). Each course of chemotherapy after recurrence increases survival, albeit with diminishing returns: OS decreases from 11.3 to 6.2 months and PFS from 6.4 to 4.4 months after progression despite three treatment regimens (281).
Treatment
Cytotoxic chemotherapy
Patients who progress or develop recurrent disease after initial chemotherapy should be considered for second-line therapy (282). However, progression despite consecutive chemotherapy regimens without clinical benefit predicts limited gains with the use of further therapy (283). There is no proven single best regimen for recurrence (284). Chemotherapy regimens for recurrent disease depend on sensitivity to platinum agents. Platinum resistant disease is defined as recurrence within 6 months of initial platinum therapy. Platinum sensitive is recurrence 6 months after the initial platinum therapy (285). PS, degree of progression, end-organ status, and pre-existing toxicities from prior chemotherapy must also be taken into account before starting second and third line regimens (283).
Platinum-sensitive disease typically continues to be responsive to platinum agents, with longer “platinum-free intervals” between therapies predictive of better response (285). Combination therapy with two agents has been shown to have better RR, OS, and PFS than monotherapy (286-288). Use of three agents, however, increases toxicity without significant OS or PFS benefit (289). Cisplatin and carboplatin are considered equivalent, although carboplatin regimens are more frequently recommended and are associated with better QOL (282,290,291). Median survival with platinum agents alone is approximately 17 to 24 months (286). Agents used in combination therapy for platinum-sensitive disease include paclitaxel, gemcitabine and pegylated liposomal doxorubicin. The paclitaxel combination has a 25% RR, 21 to 29 months OS, and 13 months PFS (287). Gemcitabine increases RR and PFS, without improving QOL or OS (292). Compared to paclitaxel, pegylated liposomal doxorubicin improves PFS by approximately 2 months without improving OS; while demonstrating more nausea, vomiting (293,294).
The current standard for platinum-resistant therapy is treatment with sequential single agents given no evidence of improved survival with combinations (286,295,296). Single agents show similar efficacy with RRs of 13-50% and OS of 6 to 16.8 months (294,297).
Targeted therapy
Bevacizumab is an option for relapsed patients in conjunction with a carboplatin/gemcitabine regimen or as monotherapy. Single agent bevacizumab has RRs of 16-21% among platinum-sensitive and platinum-resistant patients with an OS of 10.7 to 17 months (298,299). A meta-analysis in 2013 confirmed improvement upon cytotoxic chemotherapy regimens in RR by approximately 20% and PFS by 4 to 5 months among relapsed patients, albeit not OS (300).
Role of palliative care
Palliative care for patients with advanced ovarian cancer includes a multidisciplinary approach to pain, constipation or diarrhea, nausea and vomiting, anorexia, dyspnea and hypercalcemia (301). Additionally, patients frequently struggle with anxiety and depression throughout the disease process (302). Patients with advanced ovarian cancer frequently develop malignant bowel obstruction (303,304). Octreotide inhibits the secretion of multiple secretory enzymes and is effective for refractory nausea and vomiting associated with malignant bowel obstruction (305). Recurrent ascites is problematic, and may require frequent abdominal paracentesis (306). Case reports suggest that bevacizumab may help control ascites (307).
Conclusions
Optimal care for patients receiving chemotherapy for advanced cancers involves a multifaceted approach which should include oncologists in coordination with palliative care specialists. Comprehensive palliative care consists not only of symptom management and supportive care, but also longitudinal goals of care discussions as well as spiritual and psychosocial support for patients and their families throughout the trajectory of their illness. This review summarized the indications and benefits of the recommended palliative chemotherapies for some of the most common malignancies. The decision to pursue chemotherapy for patients with advanced cancer rests on prognosis, PS, benefits of therapy, QOL, clinical trial options, comorbidities, patient preferences, and symptom burden throughout the continuum of their care.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Wright AA, Zhang B, Keating NL, et al. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: prospective cohort study. BMJ (Clinical research ed) 2014;348:g1219. [PubMed]
- Sánchez-Muñoz A, Pérez-Ruiz E, Sáez MI, et al. Limited impact of palliative chemotherapy on survival in advanced solid tumours in patients with poor performance status. Clin Transl Oncol 2011;13:426-9. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg 1995;181:193-201. [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000;6 Suppl 1:S11-4. [PubMed]
- Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994;271:907-13. [PubMed]
- Ma C, Armstrong AW. Severe adverse events from the treatment of advanced melanoma: a systematic review of severe side effects associated with ipilimumab, vemurafenib, interferon alfa-2b, dacarbazine and interleukin-2. J Dermatolog Treat 2014;25:401-8. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [PubMed]
- Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol 2011;164:776-84. [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF and BRAF mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [PubMed]
- Kaufman HL, Kirkwood JM, Hodi FS, et al. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol 2013;10:588-98. [PubMed]
- Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2013;11:395-407. [PubMed]
- Eigentler TK, Caroli UM, Radny P, et al. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol 2003;4:748-59. [PubMed]
- Yang AS, Chapman PB. The history and future of chemotherapy for melanoma. Hematol Oncol Clin North Am 2009;23:583-97. [PubMed]
- Serrone L, Zeuli M, Sega FM, et al. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res 2000;19:21-34. [PubMed]
- Szynglarewicz B, Ekiert M, Forgacz J, et al. The role of surgery in the treatment of colorectal metastases from primary skin melanoma. Colorectal Dis 2012;14:e305-11. [PubMed]
- Skibber JM, Soong SJ, Austin L, et al. Cranial irradiation after surgical excision of brain metastases in melanoma patients. Ann Surg Oncol 1996;3:118-23. [PubMed]
- Fox MC, Lao CD, Schwartz JL, et al. Management options for metastatic melanoma in the era of novel therapies: a primer for the practicing dermatologist: part II: Management of stage IV disease. J Am Acad Dermatol 2013;68:13.e1-13; quiz 26-8.
- Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol 2007;2:686-93. [PubMed]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010;(5):CD007309. [PubMed]
- Lilenbaum RC, Cashy J, Hensing TA, et al. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol 2008;3:125-9. [PubMed]
- Azzoli CG, Temin S, Giaccone G. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Oncol Pract 2012;8:63-6. [PubMed]
- Socinski MA, Evans T, Gettinger S, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e341S-68S.
- National Comprehensive Cancer Network. Non-small cell lung cancer guidelines (version 3.2014). Available online: http://www.nccn.org/professionals/physician_gls/pdf/nsclc
- Dacic S. Molecular genetic testing for lung adenocarcinomas: a practical approach to clinically relevant mutations and translocations. J Clin Pathol 2013;66:870-4. [PubMed]
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432-3. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [PubMed]
- Sequist LV, Joshi VA, Janne PA, et al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist 2007;12:90-8. [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- O’Bryant CL, Wenger SD, Kim M, et al. Crizotinib: a new treatment option for ALK-positive non-small cell lung cancer. Ann Pharmacother 2013;47:189-97. [PubMed]
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [PubMed]
- Shaw AT, Camidge DR, Engelman JA, et al. Clinical activity of crizotinib in advanced non-small cell lung cancer (NSCLC) harboring ROS1 gene rearrangement. J Clin Oncol 2012;30:abstr 7508.
- Cui J, Cai X, Zhu M, et al. The efficacy of bevacizumab compared with other targeted drugs for patients with advanced NSCLC: a meta-analysis from 30 randomized controlled clinical trials. PloS one 2013;8:e62038. [PubMed]
- Lima AB, Macedo LT, Sasse AD. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. PloS one 2011;6:e22681. [PubMed]
- Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013;24:20-30. [PubMed]
- Souquet PJ, Chauvin F, Boissel JP, et al. Polychemotherapy in advanced non small cell lung cancer: a meta-analysis. Lancet 1993;342:19-21. [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008;26:4617-25. [PubMed]
- Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 1999;91:66-72. [PubMed]
- de Castria TB, da Silva EM, Gois AF, et al. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2013;8:CD009256. [PubMed]
- Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 2007;99:847-57. [PubMed]
- Rajeswaran A, Trojan A, Burnand B, et al. Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer 2008;59:1-11. [PubMed]
- Matsuda A, Yamaoka K, Tango T. Quality of life in advanced non-small cell lung cancer patients receiving palliative chemotherapy: A meta-analysis of randomized controlled trials. Exp Ther Med 2012;3:134-140. [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [PubMed]
- Dancey J, Shepherd FA, Gralla RJ, et al. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung cancer 2004;43:183-94. [PubMed]
- Pircher A, Wasle IK, Mian M, et al. Docetaxel in the treatment of non-small cell lung cancer (NSCLC) —an observational study focusing on symptom improvement. Anticancer Res 2013;33:3831-6. [PubMed]
- Li M, Zhang Q, Fu P, et al. Pemetrexed plus platinum as the first-line treatment option for advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. PloS one 2012;7:e37229. [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [PubMed]
- Grossi F, Aita M, Defferrari C, et al. Impact of third-generation drugs on the activity of first-line chemotherapy in advanced non-small cell lung cancer: a meta-analytical approach. Oncologist 2009;14:497-510. [PubMed]
- Yates P, Schofield P, Zhao I, et al. Supportive and palliative care for lung cancer patients. J Thorac Dis 2013;5:S623-8. [PubMed]
- Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80. [PubMed]
- Rossi A, Gridelli C, Ricciardi S, et al. Bone metastases and non-small cell lung cancer: from bisphosphonates to targeted therapy. Curr Med Chem 2012;19:5524-35. [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [PubMed]
- Gennari A, Conte P, Rosso R, et al. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 2005;104:1742-50. [PubMed]
- O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005;10 Suppl 3:20-9. [PubMed]
- Osborne CK, Yochmowitz MG, Knight WA 3rd, et al. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer 1980;46:2884-8. [PubMed]
- Bloom ND, Tobin EH, Schreibman B, et al. The role of progesterone receptors in the management of advanced breast cancer. Cancer 1980;45:2992-7. [PubMed]
- Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev 2003;CD002747. [PubMed]
- Ravdin PM, Green S, Dorr TM, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol 1992;10:1284-91. [PubMed]
- Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med 1998;339:1609-18. [PubMed]
- Mao C, Yang ZY, He BF, et al. Toremifene versus tamoxifen for advanced breast cancer. Cochrane Database Syst Rev 2012;7:CD008926.[PubMed]
- Gibson L, Lawrence D, Dawson C, et al. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev 2009;(4):CD003370. [PubMed]
- Lønning PE, Bajetta E, Murray R, et al. Activity of exemestane in metastatic breast cancer after failure of nonsteroidal aromatase inhibitors: a phase II trial. J Clin Oncol 2000;18:2234-44. [PubMed]
- Dombernowsky P, Smith I, Falkson G, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol 1998;16:453-61. [PubMed]
- Buzdar A, Jonat W, Howell A, et al. Anastrozole, a potent and selective aromatase inhibitor, versus megestrol acetate in postmenopausal women with advanced breast cancer: results of overview analysis of two phase III trials. Arimidex Study Group. J Clin Oncol 1996;14:2000-11. [PubMed]
- Valachis A, Mauri D, Polyzos NP, et al. Fulvestrant in the treatment of advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol 2010;73:220-7. [PubMed]
- Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol 2009;27:4530-5. [PubMed]
- Robertson JF, Lindemann JP, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat 2012;136:503-11. [PubMed]
- Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012;367:435-44. [PubMed]
- Bergh J, Jonsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 2012;30:1919-25. [PubMed]
- Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 2013;14:989-98. [PubMed]
- Wilcken N, Dear R. Chemotherapy in metastatic breast cancer: A summary of all randomised trials reported 2000-2007. Eur J Cancer 2008;44:2218-25. [PubMed]
- Gasparini G, Dal Fior S, Panizzoni GA, et al. Weekly epirubicin versus doxorubicin as second line therapy in advanced breast cancer. A randomized clinical trial. Am J Clin Oncol 1991;14:38-44. [PubMed]
- Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 2005;23:5542-51. [PubMed]
- Dear RF, McGeechan K, Jenkins MC, et al. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev 2013;12:CD008792. [PubMed]
- National Comprehensive Cancer N. Breast Cancer (Version 2.2014). Available online: www.nccn.org
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12. [PubMed]
- Harris CA, Ward RL, Dobbins TA, et al. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol 2011;22:1308-17. [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [PubMed]
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265-74. [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [PubMed]
- Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2013;31:1157-63. [PubMed]
- Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [PubMed]
- Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14:461-71. [PubMed]
- Gelmon KA, Boyle F, Kaufman B, et al. Open-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: Interim analysis (IA) of NCIC CTG MA.31/GSK EGF 108919. J Clin Oncol (ASCO Annual Meeting Abstracts) 2012;30:LBA671.
- Laird BJ, Fallon MT. Palliative care in the elderly breast cancer patient. Clin Oncol (R Coll Radiol) 2009;21:131-9. [PubMed]
- Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2012;2:CD003474. [PubMed]
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132-9. [PubMed]
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006;144:753-61. [PubMed]
- Mustafa M, Carson-Stevens A, Gillespie D, et al. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev 2013;6:CD004253. [PubMed]
- Pond GR, Sonpavde G, de Wit R, et al. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol 2014;65:3-6. [PubMed]
- Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 2007;25:1596-605. [PubMed]
- Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 2013;368:1314-25. [PubMed]
- Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med 2000;132:566-77. [PubMed]
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [PubMed]
- Ryan CJ, Smith MR, De Bono JS, et al. Interim analysis (IA) results of COU-AA-302, a randomized, phase III study of abiraterone acetate (AA) in chemotherapy-naive patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2012;30:abstract LBA 4518.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. [PubMed]
- Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 2004;22:1025-33. [PubMed]
- Taplin ME, Bubley GJ, Ko YJ, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res 1999;59:2511-5. [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [PubMed]
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. [PubMed]
- Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008;26:242-5. [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [PubMed]
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94:1458-68. [PubMed]
- Din OS, Thanvi N, Ferguson CJ, et al. Palliative prostate radiotherapy for symptomatic advanced prostate cancer. Radiother Oncol 2009;93:192-6. [PubMed]
- Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer 2004;101:2222-9. [PubMed]
- Huang SH, Perez-Ordonez B, Liu FF, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:276-83. [PubMed]
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116-27. [PubMed]
- Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2005;23:3562-7. [PubMed]
- Guardiola E, Peyrade F, Chaigneau L, et al. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer 2004;40:2071-6. [PubMed]
- Raguse JD, Gath HJ, Bier J, Riess H, Oettle H. Gemcitabine in the treatment of advanced head and neck cancer. Clin Oncol (R Coll Radiol) 2005;17:425-9. [PubMed]
- Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 2007;25:2171-7. [PubMed]
- Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol 2013;14:697-710. [PubMed]
- Mercado G, Adelstein DJ, Saxton JP, et al. Hypothyroidism: a frequent event after radiotherapy and after radiotherapy with chemotherapy for patients with head and neck carcinoma. Cancer 2001;92:2892-7. [PubMed]
- Capuano G, Gentile PC, Bianciardi F, et al. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support Care Cancer 2010;18:433-7. [PubMed]
- Hanson LC, Garrett JM, Lewis C, et al. Physicians’ expectations of benefit from tube feeding. J Palliat Med 2008;11:1130-4. [PubMed]
- De Boer MF, McCormick LK, Pruyn JF, et al. Physical and psychosocial correlates of head and neck cancer: a review of the literature. Otolaryngol Head Neck Surg 1999;120:427-36. [PubMed]
- Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg 2013;139:678-86. [PubMed]
- Scheithauer W, Rosen H, Kornek GV, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993;306:752-5. [PubMed]
- Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 2000;321:531-5. [PubMed]
- Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209-14. [PubMed]
- Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 2007;370:135-42. [PubMed]
- Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;370:143-52. [PubMed]
- Goldberg RM, Rothenberg ML, Van Cutsem E, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. The oncologist 2007;12:38-50. [PubMed]
- Peppercorn JM, Smith TJ, Helft PR, et al. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol 2011;29:755-60. [PubMed]
- Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 2012;23:2479-516. [PubMed]
- Sargent DJ, Kohne CH, Sanoff HK, et al. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol 2009;27:1948-55. [PubMed]
- National Comprehensive Cancer N. Colon Cancer (Version 3.2014). Available online: www.nccn.org
- Twelves C, Gollins S, Grieve R, et al. A randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorouracil/leucovorin regimens in patients with advanced colorectal cancer. Ann Oncol 2006;17:239-45. [PubMed]
- Cunningham D, Pyrhonen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352:1413-8. [PubMed]
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355:1041-7. [PubMed]
- Köhne CH, van Cutsem E, Wils J, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol 2005;23:4856-65. [PubMed]
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905-14. [PubMed]
- André T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer 1999;35:1343-7. [PubMed]
- Clarke SJ, Yip S, Brown C, et al. Single-agent irinotecan or FOLFIRI as second-line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta-analysis Eur J Cancer 2011;47:1826-36. [PubMed]
- Bidard FC, Tournigand C, André T, et al. Efficacy of FOLFIRI-3 (irinotecan D1,D3 combined with LV5-FU) or other irinotecan-based regimens in oxaliplatin-pretreated metastatic colorectal cancer in the GERCOR OPTIMOX1 study. Ann Oncol 2009;20:1042-7. [PubMed]
- Díaz-Rubio E, Sastre J, Zaniboni A, et al. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol 1998;9:105-8. [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30. [PubMed]
- Maindrault-Goebel F, Louvet C, Andre T, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer 1999;35:1338-42. [PubMed]
- Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006-12. [PubMed]
- Arkenau HT, Arnold D, Cassidy J, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol 2008;26:5910-7. [PubMed]
- Zhang C, Wang J, Gu H, et al. Capecitabine plus oxaliplatin compared with 5-fluorouracil plus oxaliplatin in metastatic colorectal cancer: Meta-analysis of randomized controlled trials. Oncol Lett 2012;3:831-838. [PubMed]
- Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 2011;12:642-53. [PubMed]
- Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394-400. [PubMed]
- Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [PubMed]
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866-75. [PubMed]
- Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer 2006;94:798-805. [PubMed]
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670-6. [PubMed]
- Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology 2009;77:113-9. [PubMed]
- Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005;23:3706-12. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Lv C, Wu S, Zheng D, et al. The efficacy of additional bevacizumab to cytotoxic chemotherapy regimens for the treatment of colorectal cancer: an updated meta-analysis for randomized trials. Cancer Biother Radiopharm 2013;28:501-9. [PubMed]
- Galfrascoli E, Piva S, Cinquini M, et al. Risk/benefit profile of bevacizumab in metastatic colon cancer: a systematic review and meta-analysis. Dig Liver Dis 2011;43:286-94. [PubMed]
- Ciombor KK, Berlin J. Aflibercept--a decoy VEGF receptor. Curr Oncol Rep 2014;16:368. [PubMed]
- Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [PubMed]
- Au HJ, Karapetis CS, O’Callaghan CJ, et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 Trial. J Clin Oncol 2009;27:1822-8. [PubMed]
- Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [PubMed]
- Price T, Peeters M, Kim TW, et al. ASPECCT: a randomized, multicenter, open-label, phase 3 study of panitumumab (pmab) vs cetuximab (cmab) for previously treated wild-type (WT) KRAS metastatic colorectal cancer (mCRC). Presented at: European Cancer Congress 2013; September 27-October 1, 2013; Amsterdam, The Netherlands. Abstract LBA18.
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- De Wit M, Boers-Doets CB, Saettini A, et al. Prevention and management of adverse events related to regorafenib. Support Care Cancer 2014;22:837-46. [PubMed]
- Dixon MR, Stamos MJ. Strategies for palliative care in advanced colorectal cancer. Dig Surg 2004;21:344-51. [PubMed]
- Aggarwal G, Glare P, Clarke S, et al. Palliative and shared care concepts in patients with advanced colorectal cancer. ANZ J Surg 2006;76:175-80. [PubMed]
- Nemes R, Vasile I, Curca T, et al. Acute bowel obstruction - the main complication of colorectal cancer. Therapeutical options. Rom J Gastroenterol 2004;13:109-12. [PubMed]
- Ansaloni L, Andersson RE, Bazzoli F, et al. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg 2010;5:29. [PubMed]
- van Hooft JE, Bemelman WA, Oldenburg B, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 2011;12:344-52. [PubMed]
- Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev 2011;CD007378. [PubMed]
- Lee HJ, Hong SP, Cheon JH, et al. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc 2011;73:535-42. [PubMed]
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007;21:225-33. [PubMed]
- Law WL, Choi HK, Chu KW. Comparison of stenting with emergency surgery as palliative treatment for obstructing primary left-sided colorectal cancer. Br J Surg 2003;90:1429-33. [PubMed]
- Watt AM, Faragher IG, Griffin TT, et al. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 2007;246:24-30. [PubMed]
- Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc 2010;71:560-72. [PubMed]
- Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control 2006;17:403-9. [PubMed]
- Boeck S, Hinke A, Wilkowski R, et al. Importance of performance status for treatment outcome in advanced pancreatic cancer. World J Gastroenterol 2007;13:224-7. [PubMed]
- Ueda A, Hosokawa A, Ogawa K, et al. Treatment outcome of advanced pancreatic cancer patients who are ineligible for a clinical trial. Onco Targets Ther 2013;6:491-6. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23-9. [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Petrelli F, Coinu A, Borgonovo K, et al. Polychemotherapy or gemcitabine in advanced pancreatic cancer: A meta-analysis. Dig Liver Dis 2014;46:452-9. [PubMed]
- Ciliberto D, Botta C, Correale P, et al. Role of gemcitabine-based combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomised trials. Eur J Cancer 2013;49:593-603. [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [PubMed]
- Stathopoulos GP, Syrigos K, Aravantinos G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer 2006;95:587-92. [PubMed]
- Schneider BP, Ganjoo KN, Seitz DE, et al. Phase II study of gemcitabine plus docetaxel in advanced pancreatic cancer: a Hoosier Oncology Group study. Oncology 2003;65:218-23. [PubMed]
- Stathopoulos GP, Mavroudis D, Tsavaris N, et al. Treatment of pancreatic cancer with a combination of docetaxel, gemcitabine and granulocyte colony-stimulating factor: a phase II study of the Greek Cooperative Group for Pancreatic Cancer. Ann Oncol 2001;12:101-3. [PubMed]
- Jacobs AD, Otero H, Picozzi VJ Jr, et al. Gemcitabine combined with docetaxel for the treatment of unresectable pancreatic carcinoma. Cancer Invest 2004;22:505-14. [PubMed]
- Ryan DP, Kulke MH, Fuchs CS, et al. A Phase II study of gemcitabine and docetaxel in patients with metastatic pancreatic carcinoma. Cancer 2002;94:97-103. [PubMed]
- Lutz MP, Van Cutsem E, Wagener T, et al. Docetaxel plus gemcitabine or docetaxel plus cisplatin in advanced pancreatic carcinoma: randomized phase II study 40984 of the European Organisation for Research and Treatment of Cancer Gastrointestinal Group. J Clin Oncol 2005;23:9250-6. [PubMed]
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-8. [PubMed]
- Chao Y, Wu CY, Wang JP, et al. A randomized controlled trial of gemcitabine plus cisplatin versus gemcitabine alone in the treatment of metastatic pancreatic cancer. Cancer Chemother Pharmacol 2013;72:637-42. [PubMed]
- Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002;20:3270-5. [PubMed]
- Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 2010;28:1645-51. [PubMed]
- Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [PubMed]
- Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004;22:3776-83. [PubMed]
- Kulke MH, Tempero MA, Niedzwiecki D, et al. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904. J Clin Oncol 2009;27:5506-12. [PubMed]
- Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007;25:2212-7. [PubMed]
- Rahma OE, Duffy A, Liewehr DJ, et al. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 2013;24:1972-9. [PubMed]
- Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. [PubMed]
- National Comprehensive Cancer N. Pancreatic Adenocarcinoma (Version 1.2014). Available online: www.nccn.org
- Yamanaka Y, Friess H, Kobrin MS, et al. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res 1993;13:565-9. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]
- Torgerson S, Wiebe LA. Supportive care of the patient with advanced pancreatic cancer. Oncology (Williston Park) 2013;27:183-90. [PubMed]
- Nakakura EK, Warren RS. Palliative care for patients with advanced pancreatic and biliary cancers. Surg Oncol 2007;16:293-7. [PubMed]
- van den Bosch RP, van der Schelling GP, Klinkenbijl JH, et al. Guidelines for the application of surgery and endoprostheses in the palliation of obstructive jaundice in advanced cancer of the pancreas. Ann Surg 1994;219:18-24. [PubMed]
- Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev 2006;CD004200. [PubMed]
- Glazer ES, Hornbrook MC, Krouse RS. A Meta-Analysis of Randomized Trials: Immediate Stent Placement vs. Surgical Bypass in the Palliative Management of Malignant Biliary Obstruction. J Pain Symptom Manage 2014;47:307-14. [PubMed]
- Ballinger AB, McHugh M, Catnach SM, et al. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut 1994;35:467-70. [PubMed]
- Robson PC, Heffernan N, Gonen M, et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol 2010;17:2303-11. [PubMed]
- Maire F, Hammel P, Ponsot P, et al. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol 2006;101:735-42. [PubMed]
- Tendler DA. Malignant gastric outlet obstruction: bridging another divide. Am J Gastroenterol 2002;97:4-6. [PubMed]
- Chandrasegaram MD, Eslick GD, Mansfield CO, et al. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction. Surg Endosc 2012;26:323-9. [PubMed]
- Jeurnink SM, Steyerberg EW, Hof G, et al. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol 2007;96:389-96. [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491-7. [PubMed]
- Fearon KC, Baracos VE. Cachexia in pancreatic cancer: new treatment options and measures of success. HPB (Oxford) 2010;12:323-4. [PubMed]
- Gooden HM, White KJ. Pancreatic cancer and supportive care--pancreatic exocrine insufficiency negatively impacts on quality of life. Support Care Cancer 2013;21:1835-41. [PubMed]
- Watson L. Exocrine insufficiency and pancreatic enzyme replacement therapy in pancreatic cancer. Clin Oncol (R Coll Radiol) 2010;22:391. [PubMed]
- Domínguez-Muñoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep 2007;9:116-22. [PubMed]
- Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998;42:92-6. [PubMed]
- Wyse JM, Carone M, Paquin SC, et al. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol 2011;29:3541-6. [PubMed]
- Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA 2004;291:1092-9. [PubMed]
- Clark KL, Loscalzo M, Trask PC, et al. Psychological distress in patients with pancreatic cancer--an understudied group. Psychooncology. 2010;19:1313-20. [PubMed]
- Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer. Cancer 2011;117:642-7. [PubMed]
- Boyd AD, Brown D, Henrickson C, et al. Screening for depression, sleep-related disturbances, and anxiety in patients with adenocarcinoma of the pancreas: a preliminary study. ScientificWorldJournal 2012;2012:650707.
- Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458-64. [PubMed]
- Epstein AS, O’Reilly EM. Exocrine pancreas cancer and thromboembolic events: a systematic literature review. J Natl Compr Canc Netw 2012;10:835-46. [PubMed]
- Akl EA, Labedi N, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev 2011;CD006650. [PubMed]
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53. [PubMed]
- Riess H, Pelzer U, Hilbig A, et al. Rationale and design of PROSPECT-CONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy). BMC Cancer 2008;8:361. [PubMed]
- Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004;22:2395-403. [PubMed]
- Homs MY. Chemotherapy for metastatic carcinoma of the esophagus and gastro-esophageal junction. Cochrane Database Syst Rev 2006;CD004063. [PubMed]
- Adenis A, Penel N, Horn S, et al. Palliative chemotherapy does not improve survival in metastatic esophageal cancer. Oncology 2010;79:46-54. [PubMed]
- Al-Batran SE, Ajani JA. Impact of chemotherapy on quality of life in patients with metastatic esophagogastric cancer. Cancer 2010;116:2511-8. [PubMed]
- Amdal CD, Jacobsen AB, Guren MG, et al. Patient-reported outcomes evaluating palliative radiotherapy and chemotherapy in patients with oesophageal cancer: a systematic review. Acta Oncol 2013;52:679-90. [PubMed]
- Jung HA, Adenis A, Lee J, et al. Nomogram to predict treatment outcome of fluoropyrimidine/platinum-based chemotherapy in metastatic esophageal squamous cell carcinoma. Cancer Res Treat 2013;45:285-94. [PubMed]
- Jatoi A, Foster NR, Egner JR, et al. Older versus younger patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and stomach: a pooled analysis of eight consecutive North Central Cancer Treatment Group (NCCTG) trials. Int J Oncol 2010;36:601-6. [PubMed]
- National Comprehensive Cancer N. Esophageal and Esophagogastric Junction Cancers (Version 2.2013). Available online: www.nccn.org
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [PubMed]
- Grünberger B, Raderer M, Schmidinger M, et al. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res 2007;27:2705-14. [PubMed]
- Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer 1997;33:1216-20. [PubMed]
- Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002;20:1996-2004. [PubMed]
- Enzinger PC, Ryan DP, Clark JW, et al. Weekly docetaxel, cisplatin, and irinotecan (TPC): results of a multicenter phase II trial in patients with metastatic esophagogastric cancer. Ann Oncol 2009;20:475-80. [PubMed]
- Burkart C, Bokemeyer C, Klump B, et al. A phase II trial of weekly irinotecan in cisplatin-refractory esophageal cancer. Anticancer Res 2007;27:2845-8. [PubMed]
- Ilson DH, Wadleigh RG, Leichman LP, et al. Paclitaxel given by a weekly 1-h infusion in advanced esophageal cancer. Ann Oncol 2007;18:898-902. [PubMed]
- Ajani JA, Ilson DH, Daugherty K, et al. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 1994;86:1086-91. [PubMed]
- Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol 2011;29:4709-14. [PubMed]
- Thompson SK, Sullivan TR, Davies R, et al. Her-2/neu gene amplification in esophageal adenocarcinoma and its influence on survival. Ann Surg Oncol 2011;18:2010-7. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RJ. Palliative therapy for patients with unresectable esophageal carcinoma. Surg Clin North Am 2012;92:1337-51. [PubMed]
- Shenfine J, McNamee P, Steen N, et al. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol 2009;104:1674-85. [PubMed]
- Sreedharan A, Harris K, Crellin A, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev 2009;CD005048. [PubMed]
- Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364:1497-504. [PubMed]
- Grilo A, Santos CA, Fonseca J. Percutaneous endoscopic gastrostomy for nutritional palliation of upper esophageal cancer unsuitable for esophageal stenting. Arq Gastroenterol 2012;49:227-31. [PubMed]
- Margolis M, Alexander P, Trachiotis GD, et al. Percutaneous endoscopic gastrostomy before multimodality therapy in patients with esophageal cancer. Ann Thorac Surg 2003;76:1694-7; discussion 1697-8.
- Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist 2004;9:561-70. [PubMed]
- Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95 Suppl 1:S161-92. [PubMed]
- Bonnefoi H, A’Hern RP, Fisher C, et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol 1999;17:767-75. [PubMed]
- Wimberger P, Wehling M, Lehmann N, et al. Influence of residual tumor on outcome in ovarian cancer patients with FIGO stage IV disease: an exploratory analysis of the AGO-OVAR (Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group). Ann Surg Oncol 2010;17:1642-8. [PubMed]
- Carey MS, Bacon M, Tu D, et al. The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecol Oncol 2008;108:100-5. [PubMed]
- Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol 2012;23:2605-12. [PubMed]
- National Comprehensive Cancer N. Ovarian Cancer (Version 1.2014). Available online: http://www.nccn.org/patients/guidelines/ovarian/index.html
- Griffiths RW, Zee YK, Evans S, et al. Outcomes after multiple lines of chemotherapy for platinum-resistant epithelial cancers of the ovary, peritoneum, and fallopian tube. Int J Gynecol Cancer 2011;21:58-65. [PubMed]
- Fung MF, Johnston ME, Eisenhauer EA, et al. Chemotherapy for recurrent epithelial ovarian cancer previously treated with platinum--a systematic review of the evidence from randomized trials. Eur J Gynaecol Oncol 2002;23:104-10. [PubMed]
- Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991;9:389-93. [PubMed]
- Fung-Kee-Fung M, Oliver T, Elit L, et al. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr Oncol 2007;14:195-208. [PubMed]
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 2003;361:2099-106. [PubMed]
- Raja FA, Counsell N, Colombo N, et al. Platinum versus platinum-combination chemotherapy in platinum-sensitive recurrent ovarian cancer: a meta-analysis using individual patient data. Ann Oncol 2013;24:3028-34. [PubMed]
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet 2002;360:505-15. [PubMed]
- Aabo K, Adams M, Adnitt P, et al. Chemotherapy in advanced ovarian cancer: four systematic meta-analyses of individual patient data from 37 randomized trials. Advanced Ovarian Cancer Trialists’ Group. Br J Cancer 1998;78:1479-87. [PubMed]
- Lakusta CM, Atkinson MJ, Robinson JW, et al. Quality of life in ovarian cancer patients receiving chemotherapy. Gynecol Oncol 2001;81:490-5. [PubMed]
- Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 2006;24:4699-707. [PubMed]
- Gibson JM, Alzghari S, Ahn C, et al. The role of pegylated liposomal doxorubicin in ovarian cancer: a meta-analysis of randomized clinical trials. Oncologist 2013;18:1022-31. [PubMed]
- Lawrie TA, Bryant A, Cameron A, et al. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst Rev 2013;7:CD006910. [PubMed]
- González-Martín A, Geico Group. Is combination chemotherapy superior to single-agent chemotherapy in second-line treatment? Int J Gynecol Cancer 2003;13 Suppl 2:185-91. [PubMed]
- Elit L, Hirte H. Palliative systemic therapy for women with recurrent epithelial ovarian cancer: current options. Onco Targets Ther 2013;6:107-18. [PubMed]
- Peng LH, Chen XY, Wu TX. Topotecan for ovarian cancer. Cochrane Database Syst Rev 2008;CD005589. [PubMed]
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473-83. [PubMed]
- Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 2007;25:5180-6. [PubMed]
- Zhou M, Yu P, Qu X, et al. Phase III Trials of Standard Chemotherapy with or without Bevacizumab for Ovarian Cancer: A Meta-Analysis. PloS one 2013;8:e81858. [PubMed]
- Rezk Y, Timmins PF 3rd, Smith HS. Review article: palliative care in gynecologic oncology. Am J Hosp Palliat Care 2011;28:356-74. [PubMed]
- Beesley VL, Price MA, Webb PM, et al. Changes in supportive care needs after first-line treatment for ovarian cancer: identifying care priorities and risk factors for future unmet needs. Psychooncology 2013;22:1565-71. [PubMed]
- Abu-Rustum NR, Barakat RR, Venkatraman E, et al. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997;64:493-5. [PubMed]
- Kucukmetin A, Naik R, Galaal K, et al. Palliative surgery versus medical management for bowel obstruction in ovarian cancer. Cochrane Database Syst Rev 2010;CD007792. [PubMed]
- Mercadante S. The role of octreotide in palliative care. J Pain Symptom Manage 1994;9:406-11. [PubMed]
- Woopen H, Sehouli J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res 2009;29:3353-9. [PubMed]
- Kobold S, Hegewisch-Becker S, Oechsle K, et al. Intraperitoneal VEGF inhibition using bevacizumab: a potential approach for the symptomatic treatment of malignant ascites? Oncologist 2009;14:1242-51. [PubMed]