The efficacy and safety of capecitabine-based versus S-1-based chemotherapy for metastatic or recurrent gastric cancer: a systematic review and meta-analysis of clinical randomized trials
Introduction
Gastric cancer (GC), particularly the unresectable, metastatic, or recurrent type, has been characterized by unfavorable prognosis. According to the 2018 Global Cancer Statistics (1), the incidence and mortality of GC ranked fifth and third in the general population, respectively. Although various therapeutic approaches, like palliative surgery, cytotoxic therapy, targeted therapy, and immunotherapy, have been applied in GC treatment, the 5-year survival rate is unsatisfactory. The National Comprehensive Cancer Network (NCCN) and the guidelines from Europe recommend fluoropyrimidine (fluorouracil or capecitabine) plus a platinum compound (cisplatin or oxaliplatin) as the first-line chemotherapy of metastatic or recurrent GC (2,3). In contrast, the Korean guideline recommends S-1, an oral fluoropyrimidine, as a safe alternative to 5-Fluorouracil (5-Fu) (4). Moreover, S-1 plus cisplatin (SP) has been suggested to be the best regimen for patients with unresectable or recurrent GC in Japan (5). However, S-1 has not been incorporated into the first choice of GC treatment in China (6). Thus, it seems that S-1 and capecitabine in the GC therapeutic field are inconsistently valued across different regions. Two meta-analyses showed that there was no significant difference in objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) between S-1 and capecitabine-based chemotherapy, but a lower incidence of HFS was observed in the S-1 groups (7,8). In the study of Ye et al. (9), the 1-year OS and 1-year PFS between S-1-based and capecitabine-based chemotherapy were similar, but the ORR and adverse events like all-grade neutropenia and HFS were significantly different.
The prevailing evidence on the efficacy and safety of S-1 and capecitabine in the treatment of GC is acquired from patients with different stages of the disease, and the efficacy and safety of these treatments in patients with metastasis or recurrent GC are unclear. High-quality meta-analyses have been recognized as one of the key tools for acquiring reliable evidence for improving disease treatment (10-12). To this end, we conducted this meta-analysis of the published clinical randomized controlled trials (RCTs) relevant to the treatment of GC.
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (13,14).
Search strategy
Two authors (Z Feng and X Hou) independently used the following search terms to identify relevant studies: “[gastric cancer OR stomach cancer OR gastric carcinoma OR stomach carcinoma OR gastric neoplasm OR stomach neoplasma] AND [S-1 OR tegafur OR FT207 OR utefos OR futraful OR sunfural OR uftoral OR florafur OR ftorafur OR BMS247616] AND [capecitabine OR xeloda]” from the PubMed, Embase, Web of Science, and Cochrane Library databases from inception to June 20th, 2019. The search was not restricted in any other way. The references of any literature found were screened to identify additional studies. Other studies were retrieved by manual searching of relevant journals.
Inclusion and exclusion criteria
We included RCTs that met the following inclusion criteria: (I) patients: adults who were diagnosed with metastatic or recurrent GC; (II) intervention: 1 treatment group receiving capecitabine-based therapy and the other receiving S-1-based therapy; (III) comparison: a capecitabine-based treatment group; (IV) outcomes: efficacy and safety data including ORR, OS, PFS, and adverse events were recorded; (V) language: published in English.
We excluded the following literature: (I) case reports, editorials, reviews and letters, animal studies, and conference papers; (II) studies with incomplete data; (III) repeated publications from the identical population or data used consistently (in such cases, only the report on the largest sample was included in this study).
Data extraction
The following information was extracted: (I) the first author and the year of publication; (II) the sample size, age, gender, and therapeutic regimen; (III) ORR, OS, and PFS; (IV) adverse events. Two reviewers (Z Feng and P Yan) independently conducted literature screening, data extraction, and quality assessment of the trials. When reviewers had a disagreement, a third reviewer (J Feng) intervened until a consensus was achieved.
Primary and secondary endpoints
The primary endpoint of the meta-analysis was efficacy, including ORR, PFS, and OS. The rate of adverse events, such as anemia, neutropenia, thrombocytopenia, anorexia, asthenia, diarrhea, HFS, and stomatitis, were regarded as secondary endpoints.
Assessment of publication biases
The risk of bias in the included RCTs was assessed according to the Cochrane Handbook version 5.1.0. The biases included detection bias, selection bias, reporting bias, performance bias, attrition bias, and other potential biases. The methodological quality was classified as having a low, high, or unclear risk of bias. The risk of bias assessment was completed independently by two reviewers, and any conflicts were resolved by a third reviewer (15-17).
Statistical analysis
The pooled estimates for dichotomous variables were reported as hazard ratio (HR), or risk ratio (RR) with 95% confidence intervals (CIs) and the results were presented as Forest plots. If no events were reported for one group in comparison, a value of 0.5 was added to both groups for each study (18). Based on the recommendation of the Cochrane Handbook (16), trials with no events in both groups were not included in the meta-analysis when RRs were calculated.
If a study did not report the OS or PFS data, all data were extracted from Kaplan-Meier survival curves by utilizing the software Engauge Digitizer (version 4.1, http://sourceforge.net/projects/digitizer). A random-effects model was applied to all pooled results. Heterogeneity was estimated based on the I2 statistic. I2 <50% indicated low heterogeneity, whereas I2 ≥50% denoted high heterogeneity. This meta-analysis was implemented using the R software (version 3.5.1, https://cran.r-project.org/) and RevMan 5 software (version 5.0.25, http://ims.cochrane.org/revman/download). All P values were two-tailed, and P values <0.05 were considered statistically significant.
Subgroup meta-analyses were conducted to investigate any potential sources of heterogeneity among studies. To explore the stability of this meta-analysis, we performed a sensitivity analysis by sequentially omitting individual studies. Finally, the funnel plots and the Egger’s and Begg’s tests were used for examining the potential for publication bias.
Results
Study selection
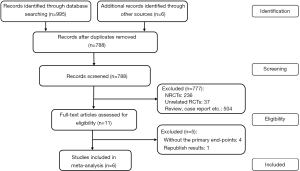
Study selection is shown in the flow chart in Figure 1. A total of 1,001 relevant studies were identified. After checking duplicated records and reviewing their titles and abstracts, 990 studies were excluded. The remaining 11 studies were assessed by full-text review. Ultimately, 6 studies were included.
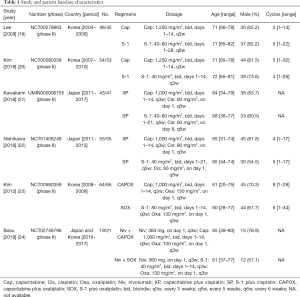
Basic characteristics of the eligible studies
The characteristics of the eligible studies are summarized in Table 1. Two studies compared capecitabine monotherapy to S-1 monotherapy (19,20), another two studies compared capecitabine plus cisplatin (XP) to S-1 plus cisplatin (SP) (21,22), one study compared capecitabine plus oxaliplatin (CAPOX) to S-1 plus oxaliplatin (SOX) (23), and one study compared nivolumab plus capecitabine plus oxaliplatin (Niv + CAPOX) to nivolumab plus S-1 plus oxaliplatin (Niv + SOX) (24). All eligible studies were conducted in Japan or Korea.
Full table
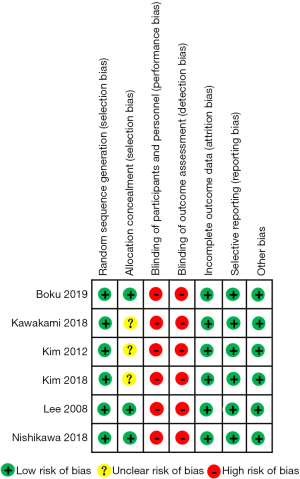
Risk of bias in individual studies
Among the six RCTs, the risk of bias was high due to the lack of blinding of participants, study personnel, and outcome assessment. Information on random sequence generation and allocation concealment were unclear in three of these studies. A summary of the proportion of trials with low, unclear, and high bias in each domain is shown in Figure 2.
A meta-analysis of ORR
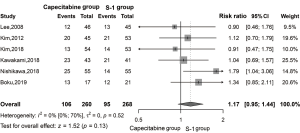
ORR was identified in six studies. Based on the random-effects model analysis, we found no significant (Figure 3) difference between the two treatment groups (RR =1.17, 95% CI: 0.95–1.44, P=0.13, I2 =0%).
A meta-analysis of PFS
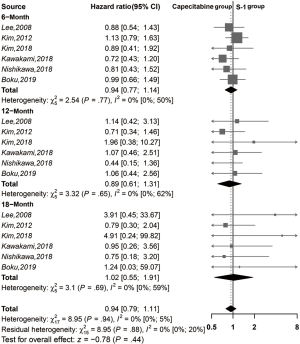
The meta-analysis showed no significant differences (Figure 4) between the two groups in terms of 6-, 12-, and 18-month PFS (RR =0.94, 95% CI: 0.77–1.14, I2 =0%; RR =0.89, 95% CI: 0.61–1.31, I2 =0%; RR =1.02, 95% CI: 0.55–1.91, I2 =0%; respectively).
A meta-analysis of OS
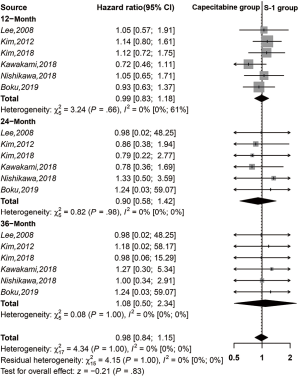
There were no significant differences between the two treatment groups in a meta-analysis of 1-, 2-, and 3-year OS (HR =0.99, 95% CI: 0.83–1.18, I2 =0%; HR =0.90, 95% CI: 0.58–1.42, I2 =0%; HR =1.08, 95% CI: 0.50–2.34, I2 =0%; respectively) (Figure 5).
A meta-analysis of adverse events
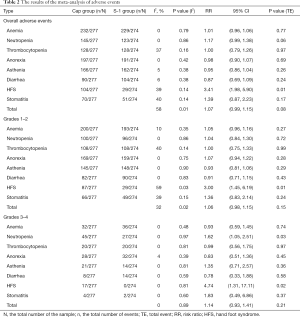
The meta-analysis of adverse events among the eligible trials is presented in Table 2. Anemia and anorexia were the most common toxicities in both groups. No significant difference was found in total adverse events between the groups (RR =1.07, 95% CI: 0.99–1.15, P=0.08, I2 =58%), but the incidence of HFS in the capecitabine-based group was higher than that of the S-1-based group (RR =3.41, 95% CI: 1.98–5.90, P<0.01, I2 =39%).
Full table
Subgroup analysis
Subgroup analyses (Table 2) for grade 1–2 and grade 3–4 adverse events indicated that the incidence of HFS was also increased in the capecitabine-based group (RR =3.00, 95% CI: 1.45–6.19, I2 =59%, P<0.01; RR =4.74, 95% CI: 1.31–17.11, I2 =0%, P=0.02; respectively). In addition, analysis of grade 3–4 adverse events showed a higher incidence of neutropenia (RR =1.62, 95% CI: 1.05–2.51, I2 =0%, P=0.03) in the capecitabine-based group. No statistically significant differences were found in other adverse events between the two treatment groups.
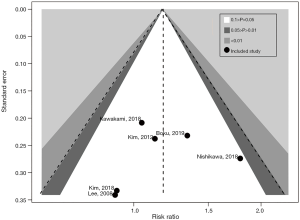
Publication bias and sensitivity analysis
The results of the sensitivity analysis showed that the present meta-analysis is stable, as revealed by the asymmetrical shape of the funnel plot (Figure 6). Furthermore, formal tests showed no substantial publication bias (P=0.695 for the Egger’s test; P=0.347 for the Begg’s test). The final results were not significantly influenced by a single study (Table 3). This suggests that the conclusions of the meta-analysis are robust.
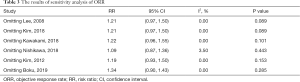
Full table
Discussion
The principal findings of this study were the following: (I) between the capecitabine-based and S-1-based chemotherapy for the patients with metastasis or recurrent GC, and there were no significant differences in ORR; 6-, 12-, and 18-month PFS; and 1-, 2-, and 3-year OS. (II) Compared to S-1-based chemotherapy, patients treated with capecitabine-based had significantly higher incidences of all-grades HFS and grades 3–4 neutropenia, but there was no significant difference in total adverse events between the two treatment groups.
Capecitabine and S-1 are both oral substitutes for 5-Fu. Capecitabine has a proven selection towards tumor cells and thus has potentially significant anti-tumor activity along with lower toxicity. It is therefore considered to be highly effective for patients with recurrent or metastatic GC in Western countries (25). Several studies showed that capecitabine-based chemotherapy is as effective as 5-Fu-based for improving prognosis and reducing recurrence, while it exhibits a higher incidence of HFS (26,27). S-1 is composed of tegafur, gimeracil and oteracil. Gimeracil saves the active form of S-1 by inhibiting dihydropyrimidine dehydrogenase, and oteracil decreases the phosphorylation of 5-Fu through combining orotate phosphoribosyltransferase in the gastrointestinal tract, all of which renders S-1 highly effective with low toxicity (28). Clinical trials on S-1 regimens in Japan had revealed that S-1 has a demonstrable effect on GC (29).
Compared to 5-Fu, S-1-based chemotherapy was superior in terms of drug-disease response rate, PFS, and OS for patients with GC (30-32). Meanwhile, S-1-based chemotherapy in GC was satisfactory in East Asia (20,33-38), but numerous investigators in Western countries questioned the benefits of this treatment as S-1 was found to have higher toxicity even at low doses for patients with advanced metastatic disease in Western trials (39). This discrepancy might be explained by the difference of gene polymorphisms encoding drug-metabolizing enzymes between Asian and Western populations (40).
RCTs and meta-analyses have been performed for identifying the better outcomes in treating GC between capecitabine-based and S-1-based regimens (7,8,19-24,41-43). However, there is no consistent conclusion from these studies. One meta-analysis pointed out that, compared to capecitabine-based therapy, S-1-based chemotherapy was associated with similar anti-tumor efficacy and a better safety profile (42). However, another meta-analysis indicated that S-1 was not as effective as capecitabine in the treatment of GC (43). A systematic review and meta-analysis of six RCTs (one of RCTs was published in Chinese) and two retrospective studies of capecitabine-based and S-1-based regimens treatments for GC reported that S-1-based therapy might be a better choice for advanced GC patients due to the higher incidences of HFS and neutropenia in capecitabine-based therapy (9).
The longest time of efficacy evaluation, for the comparison between capecitabine-based and S-1-based therapy, was within 1 year of the currently published meta-analysis. Thus, we conducted the present meta-analysis to investigate a longer period of PFS and OS and to gain more evidence on drug-related efficacy. The result of our meta-analysis suggested that there was no difference in ORR between capecitabine-based and S-1-based therapy, which revealed that both regimens could be used as first-line therapy for the patients with metastatic or recurrent GC among Asian populations. PFS did reflect the progression of the lesion earlier. However, unlike PFS, OS was not as effective in observing changes in disease over time (44). Undoubtedly, longer periods of PFS and OS were better manifestations of optimal clinical benefits for GC patients. We thus analyzed the 6-, 12-, 18-month PFS, and the 1-, 2-, 3-year OS, respectively. As to the longest-follow-up PFS or the shortest-follow-up PFS, no significant differences were found between the two treatment groups. Similar results were found for the longest-follow-up OS and the shortest-follow-up OS. Taken together, these results indicate that capecitabine-based and S-1-based regimens have similarly efficacy for the treatment of GC.
In the overall analysis of adverse events, our results showed that there was no significant difference between capecitabine-based and S-1-based therapy. In the subgroup analysis, we analyzed eight types of the most common adverse events according to the grade-level from six studies and found that the incidence of all grades HFS and grades 3–4 neutropenia in GC patients treated with capecitabine-based therapy was higher than that among patients treated with S-1-based therapy. HFS is the most common non-hematological toxicity caused by capecitabine, and adversely affects the quality of life while decreasing the efficacy of treatments. Although urea cream and celecoxib are effective in treating HFS, further studies are needed to develop more specific medicines (45,46). Therefore, according to our results, S-1-based chemotherapy displayed high efficacy for high-risk patients with HFS and intolerable hematological toxicity.
Heterogeneity is an important factor that affects the results of a meta-analysis. Since heterogeneity could not be completely ruled out in this study, a sensitivity analysis was performed to assess the robusticity of our findings. We found that no study affected the overall significance of the pooled estimates, and thus our findings are robust. Publication bias can introduce false-positive results in a meta-analysis. To evade possible biases, all the studies included were thoroughly assessed. Egger’s and Begg’s tests were performed to detect publication bias and no significant bias was found. These analyses of publication bias and sensitivity indicated that the conclusions of our study are solid.
There are several limitations to our research. Firstly, only 6 phase II trials with 561 individuals were included; therefore, additional high-quality RCTs are needed to validate the findings of this study better. Secondly, all studies are open-label, which might thus influence the outcomes. Thirdly, patients included in all studies were all from Asia, and hence the conclusions may not be generalizable to Western populations. Lastly, HRs and 95% CI, which were extracted from the Kaplan-Meier survival curves, might have influenced the pooled results.
Conclusions
In terms of efficacy, capecitabine-based chemotherapy and S-1-based chemotherapy have similar short- and medium-term outcomes. In terms of safety, we recommend S-1-based therapy as a top-priority regimen for patients with metastatic or recurrent GC.
Acknowledgments
Funding: This study was supported by the Key Laboratory of Evidence-Based Medicine and Knowledge Translation Foundation of Gansu Province (grant number: GSXZYZH2018006).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.04.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Pennsylvania: NCCN, 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 2019;30:19-33. [Crossref] [PubMed]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA). Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer 2019;19:1-48. [Crossref] [PubMed]
- Liang H. The Precised Management of Surgical Treatment for Gastric Cancer: Interpretation of the 5th edition of Japanese Gastric Cancer Treatment Guideline and the 15th edition of Japanese Classification for Gastric Cancer. Zhonghua Zhong Liu Za Zhi 2019;41:168-72.
- Wang FH, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. [Crossref] [PubMed]
- He AB, Peng XL, Song J, et al. Efficacy of S-1 vs capecitabine for the treatment of gastric cancer: a meta-analysis. World J Gastroenterol 2015;21:4358-64. [Crossref] [PubMed]
- Zhang X, Cao C, Zhang Q, et al. Comparison of the efficacy and safety of S-1-based and capecitabine-based regimens in gastrointestinal cancer: a meta-analysis. PLoS One 2014;9:e84230. [Crossref] [PubMed]
- Ye Z, Chen J, Rao Y, et al. Should S-1 be better than capecitabine for patients with advanced gastric cancer in Asia? A systematic review and meta-analysis. Onco Targets Ther 2018;12:269-77. [Crossref] [PubMed]
- Tian J, Zhang J, Ge L, et al. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol 2017;85:50-8. [Crossref] [PubMed]
- Yan P, Yao L, Li H, et al. The methodological quality of robotic surgical meta-analyses needed to be improved: a cross-sectional study. J Clin Epidemiol 2019;109:20-9. [Crossref] [PubMed]
- Yao L, Sun R, Chen YL, et al. The quality of evidence in Chinese meta-analyses needs to be improved. J Clin Epidemiol 2016;74:73-9. [Crossref] [PubMed]
- Wang X, Chen Y, Yao L, et al. Reporting of declarations and conflicts of interest in WHO guidelines can be further improved. J Clin Epidemiol 2018;98:1-8. [Crossref] [PubMed]
- Ge L, Tian JH, Li YN, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol 2018;93:45-55. [Crossref] [PubMed]
- Nakamura Y, Yamanaka T, Chin K, et al. Survival outcomes of two phase 2 studies of adjuvant chemotherapy with S-1 plus oxaliplatin or capecitabine plus oxaliplatin for patients with gastric cancer after D2 gastrectomy. Ann Surg Oncol 2019;26:465-72. [Crossref] [PubMed]
- Higgins JPT, Green S. editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. London: The Cochrane Collaboration, 2011. Available online: http://www.cochrane-handbook.org
- Pan B, Ge L, Xun YQ, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act 2018;15:72. [Crossref] [PubMed]
- Zhao JG, Zeng XT, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA 2017;318:2466-82. [Crossref] [PubMed]
- Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 2008;99:584-90. [Crossref] [PubMed]
- Kim MJ, Kong SY, Nam BH, et al. A randomized phase II study of S-1 versus capecitabine as first-line chemotherapy in elderly metastatic gastric cancer patients with or without poor performance status: clinical and pharmacogenetic results. Pharmacogenet Genomics 2018;28:23-30. [Crossref] [PubMed]
- Kawakami H, Takeno A, Endo S, et al. Randomized, open-label phase II study comparing capecitabine-cisplatin every 3 weeks with S-1-cisplatin every 5 weeks in chemotherapy-naïve patients with HER2-negative advanced gastric cancer: OGSG1105, HERBIS-4A trial. Oncologist 2018;23:1411-e147. [Crossref] [PubMed]
- Nishikawa K, Tsuburaya A, Yoshikawa T, et al. A randomised phase II trial of capecitabine plus cisplatin versus S-1 plus cisplatin as a first-line treatment for advanced gastric cancer: Capecitabine plus cisplatin ascertainment versus S-1 plus cisplatin randomised PII trial (XParTS II). Eur J Cancer 2018;101:220-8. [Crossref] [PubMed]
- Kim GM, Jeung HC, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 2012;48:518-26. [Crossref] [PubMed]
- Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019;30:250-8. [Crossref] [PubMed]
- Kim TY, Oh DY, Bang YJ. Capecitabine for the treatment of gastric cancer. Expert Rev Gastroenterol Hepatol 2015;9:1471-81. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Cunningham D, Chua YJ. East meets west in the treatment of gastric cancer. N Engl J Med 2007;357:1863-5. [Crossref] [PubMed]
- Hironaka S, Sugimoto N, Yamaguchi K, et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:99-108. [Crossref] [PubMed]
- Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063-9. [Crossref] [PubMed]
- Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886-93. [Crossref] [PubMed]
- Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 2010;28:1547-53. [Crossref] [PubMed]
- Chen X, Li W, Sun L, et al. S-l combined with cisplatin plus concurrent chemoradiotherapy versus cisplatin plus concurrent chemoradiotherapy for Chinese patients with advanced gastric cancer: a multi-centre randomized controlled trial. Clin Transl Oncol 2016;18:672-6. [Crossref] [PubMed]
- Tanaka H, Kanda M, Morita S, et al. Randomized phase II study of daily and alternate-day administration of S-1 for advanced gastric cancer (JFMC43-1003). Int J Clin Oncol 2017;22:1052-9. [Crossref] [PubMed]
- Zhao Q, Li Y, Huang J, et al. Short-term curative effect of S-1 plus oxaliplatin as perioperative chemotherapy for locally advanced gastric cancer: a prospective comparison study. Pharmazie 2017;72:236-40. [PubMed]
- Kim HS, Ryu MH, Zang DY, et al. Phase II study of oxaliplatin, irinotecan and S-1 therapy in patients with advanced gastric cancer: the Korean Cancer Study Group ST14-11. Gastric Cancer 2018;21:802-10. [Crossref] [PubMed]
- Kagawa S, Muraoka A, Kambara T, et al. A multi-institution phase II study of docetaxel and S-1 in combination with trastuzumab for HER2-positive advanced gastric cancer (DASH study). Cancer Chemother Pharmacol 2018;81:387-92. [Crossref] [PubMed]
- Wang G, Zhao J, Song Y, et al. Phase II study of adjuvant chemotherapy with S1 plus oxaliplatin for Chinese patients with gastric cancer. BMC Cancer 2018;18:547. [Crossref] [PubMed]
- Ilson DH. Current progress in the adjuvant treatment of gastric cancer. Surg Oncol Clin N Am 2017;26:225-39. [Crossref] [PubMed]
- Ma BB, Hui EP, Mok TS. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol 2010;11:75-84. [Crossref] [PubMed]
- Xue K, Ying X, Bu Z, et al. Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II-III randomized trial. Chin J Cancer Res 2018;30:516-25. [Crossref] [PubMed]
- He MM, Wu WJ, Wang F, et al. S-1-based chemotherapy versus capecitabine-based chemotherapy as first-line treatment for advanced gastric carcinoma: a meta-analysis. PLoS One 2013;8:e82798. [Crossref] [PubMed]
- Ter Veer E, Ngai LL, Valkenhoef GV, et al. Capecitabine, 5-fluorouracil and S-1 based regimens for previously untreated advanced oesophagogastric cancer: A network meta-analysis. Sci Rep 2017;7:7142. [Crossref] [PubMed]
- Tong M, Wang J, Zhang H, et al. Efficacy and safety of gemcitabine plus anti-angiogenesis therapy for advanced pancreatic cancer: a systematic review and meta-analysis of clinical randomized phase III trials. J Cancer 2019;10:968-78. [Crossref] [PubMed]
- Zhang RX, Wu XJ, Wan DS, et al. Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial. Ann Oncol 2012;23:1348-53. [Crossref] [PubMed]
- Hofheinz RD, Gencer D, Schulz H, et al. Mapisal versus urea cream as prophylaxis for capecitabine-associated hand-foot syndrome: a randomized phase III trial of the AIO Quality of Life Working Group. J Clin Oncol 2015;33:2444-9. [Crossref] [PubMed]