Enhanced recovery after surgery (ERAS) might be a standard care in radical prostatectomy: a systematic review and meta-analysis
Introduction
Prostate cancer (Pca) is the most common genitourinary tract tumor in men (1). Radical prostatectomy (RP) is a first-line treatment for low and intermediate-risk localized Pca patients (2). RP can be either open or minimally invasive. Currently, minimally invasive RPs are widely applied. These are laparoscopic radical prostatectomy (LRP) and robot-assisted laparoscopic radical prostatectomy (RALP). Particularly, RALP has become the mainstay invasive treatment for localized Pca. Compared to open RP, minimally invasive RP is an effective and widely accepted treatment for localized Pca (3-5). Moreover, it reduces perioperative outcomes such as blood loss (6). However, open RP is still an option in some countries.
Enhanced recovery after surgery (ERAS) is a protocol aimed to reduce perioperative complications as well as the physical and psychological stress of surgical trauma. It is also described as fast-track surgery (FTS), because it accelerates patient rehabilitation, shortens the hospitalization period and reduce the medical costs. It was first performed by Kehlet in 1997 (7). It includes a series of evidence-based procedures, such as surgical, nursing, medical, anesthetic and perioperative managements (8).
A high number of Pca patients are elderly people. As such, comorbidities are very common thus necessitating the reduction of perioperative complications and acceleration of patient’s recovery. ERAS has been widely applied in patients undergoing colorectal, breast and gastrointestinal surgery (9-12). However, its application in patients undergoing urological surgery is relatively low (13). To date, there is no meta-analysis to compare the efficacy and safety of ERAS to conventional care in patients undergoing RP. With more data available currently, a systematic review and meta-analysis was performed to assess whether ERAS should be considered as a standard care for patients undergoing RP.
Methods
Search strategy
We performed a comprehensive literature search on Embase, PubMed, the Clinicaltrials.gov (http://clinicaltrials.gov/), the Cochrane Library and CNKI Library to identify clinical trials that compared ERAS and conventional care. The search was done in all data published before May 2019. The search terms included: “ERAS”, “Enhanced recovery after surgery”, “Enhanced recovery”, “perioperative management”, “Fast-track surgery”, “FTS”, “Radical prostatectomy” and “Prostate cancer”. We also screened the reference lists of review articles. Additional studies were also retrieved by manual search in relevant journals. We exclusively included studies which were published in English and Chinese.
Inclusion and exclusion criteria
Studies were selected according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14). Clinical trials that met the four listed criteria were included:
- Randomized phase II, III, and IV trials;
- Patients who underwent RP;
- Participants who had received ERAS compared to conventional care and
- Trails with available events, event rates and sample sizes to enable determination of efficacy and safety of ERAS.
Trials were excluded if:
- They involved animal research;
- They were reviews only;
- Had abstracts only;
- Had overlapping data and
- Those studies without standard mean difference (SMD), risk ratio (RR), odds ratio (OR), hazard ratio (HR) and 95% confidence intervals (CIs).
Data extraction and quality assessment
Two reviewers independently did literature screening, data extraction, and quality assessment of the trials. A third reviewer was involved to have a consensus were the two reviewers disagreed. From each article, the first author’s name, year of publication, study type, disease type, the number of patients, trial phase, treatment and control arms, the number of patients with intraoperative outcomes, postoperative outcomes and postoperative complications were extracted. The quality of the methodology used in randomized controlled trials (RCTs) was determined by the Jadad criteria (15). The quality of each trial was scored and grouped as either high- or low-quality trial. High-quality trials had scores of more than three (score ≥3) while the low-quality trials had scores of less than two (score ≤2). The Newcastle-Ottawa Scale (NOS) criteria (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to determine the quality of the methodology used in non-randomized trials. Score ranged from 0 to 9 stars. High-quality trials were those with scores of more than seven stars (score ≥7 stars).
Statistical analysis
Data of patients with intraoperative outcomes, postoperative outcomes and postoperative complications was extracted from all the included trials. SMD, RR and 95% CI were calculated to determine the association strength of these two regimens with the outcomes. The Q and I2 statistics were used to determine the heterogeneity. I2 of more than fifty percent (I2>50%) indicated a statistically significant heterogeneity. The random-effect model was used in meta-analyses of the conservative statistics. Subgroup analysis was carried out based on the clinical characteristics. A funnel plot was used to determine any bias in the publications. Begg adjusted rank correlation test (16) and Egger regression test (17) were also performed to assess the publication bias. A sensitivity analysis was performed to determine whether the results of the meta-analysis were robust. STATA statistical version 12.0 software was used to perform all the statistical analyses (Stata Corporation, College Station, Texas, USA). A P value of less than 0.05 (P<0.05) was considered statistically significant. All the P vales were two-sided.
Results
Characteristics of studies included in this study
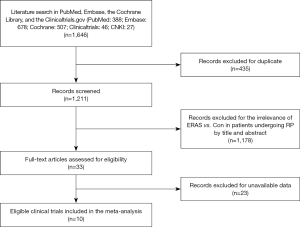
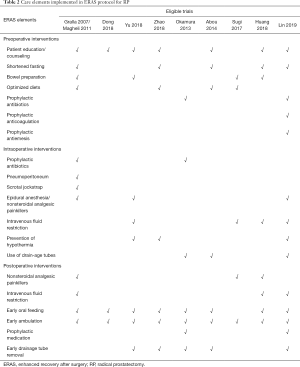
Our initial search yielded 1,646 potentially relevant clinical trials on ERAS or conventional care in patients who underwent RP. After reviewing and screening, 10 primary studies (18-27) met our inclusion criteria. Among the ten, five were RCTs studies, four were retrospective trials and one was a prospective cohort study. The studies had 3,803 subjects that were pooled for meta-analyses (Figure 1). The baseline characteristics of each trial are shown in Table 1 while the Care elements implemented in ERAS protocol for RP in each trial are shown in Table 2. All trials included were open label and had between 50 and 2,610 patients enrolled for the trial. The Jadad quality scores of the included RCTs ranged from 2 to 3 while the NOS quality scores for the prospective cohort study and the retrospective trials ranged from 7 to 8 stars. Base on the eligibility criteria of most of the trials, patients with impaired hepatic, renal or bone marrow function were excluded. A majority of the patients in these trials had an Eastern Cooperative Oncology Group (ECOG) performance-status scores of 0 or 1. This systematic review followed the guidelines of the PRISMA statement.

Full table
Full table
Findings—intraoperative outcomes (operation time and blood loss)
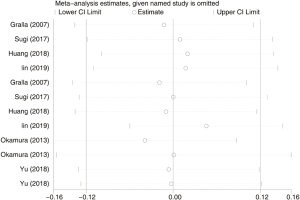
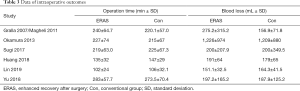
A total of 3,270 subjects treated with either ERAS or conventional care in six trials were included for the analysis of operation time and blood loss (data shown in Tables 3,4). An SMD of 0.00 (95% CI: −0.19, 0.20, I2=63.4%) were obtained from analysis of the operation times of patients under ERAS and conventional care. In the analysis of blood loss, an SMD of −0.00 (95% CI: −0.19, 0.19, I2=60.2%) were obtained (Figure 2). These results showed no statistical difference in both the operation time and blood loss between ERAS and conventional care groups (P=0.987).
Full table
Full table
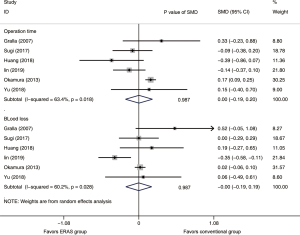
Although the results of intraoperative outcomes indicated statistically significant heterogeneity, the sensitivity analysis showed that the results of intraoperative outcomes were robust (Figure S1).
Findings—postoperative outcomes (hospital stay, catheter stay, first defecation and first anal exhaust)
Hospital stay
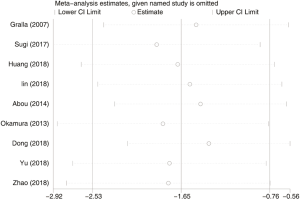
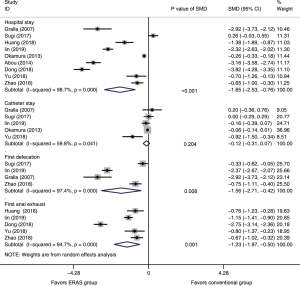
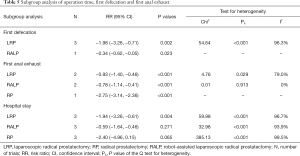
A total of 3,803 subjects treated with either ERAS or conventional care in nine trials were included for the analysis of hospital stay (data shown in Tables 3,4). An SMD of −1.65 (95% CI: −2.53, −0.76, I2=98.7%) was obtained from analysis of hospital stay of patients under ERAS and conventional care (Figure 3). The results showed that ERAS group had a shorter hospital stay than conventional care groups (P<0.001). As the results indicated statistically significant heterogeneity, subgroup analysis was performed to find sources of heterogeneity. The nine trials were first separated in three groups (LRP, RALP and RP) based on their surgery sub-type. However, the results still showed heterogeneity (Table 5) indicating that the difference in surgery sub-types was not the source of heterogeneity. However, the sensitivity analysis showed that the results of hospital stay were robust (Figure S2).
Full table
Catheter stay
A total of 3,197 subjects treated with either ERAS or conventional care in five trials were included for the analysis of catheter stay (data shown in Tables 3,4). In the analysis of catheter stay, an SMD of −0.12 (95% CI: −0.31, 0.07, I2=59.8%) were obtained (Figure 3). The results showed no statistical difference in catheter stay between ERAS and conventional care groups (P=0.204).
First defecation
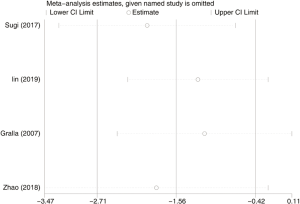
Six hundred and seventy subjects treated with either ERAS or conventional care in four trials were included for the analysis of first defecation (data shown in Tables 3,4). An SMD of −1.56 (95% CI: −2.71, −0.42, I2=97.4%) were obtained (Figure 3). The results showed that ERAS groups had a shorter time to first defecation compared to the conventional care groups (P=0.008). The results indicated statistically significant heterogeneity. Subgroup analysis was performed to find sources of heterogeneity. The four trials were first separated in two groups (LRP and RALP) based on their surgery sub-type. However, the results still showed heterogeneity (Table 5) indicating that the difference in surgery sub-types was not the source of heterogeneity. But sensitivity analysis showed that the results of first defecation were robust (Figure S3).
First anal exhaust
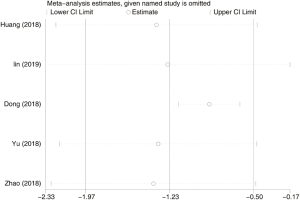
Seven hundred and forty-eight subjects treated with either ERAS or conventional care in 5 trials were included for the analysis of first anal exhaust (data shown in Tables 3,4). An SMD of −1.23 (95% CI: −1.97, −0.50, I2=94.7%) were obtained (Figure 3). The results showed that ERAS group had a shorter time to first anal exhaust than conventional care groups (P=0.001). The results indicated statistically significant heterogeneity. Subgroup analysis was performed to find sources of heterogeneity. The five trials were first separated in three groups (LRP, RALP and RP) based on their surgery sub-type. However, the results still showed heterogeneity (Table 5) indicating that the difference in surgery sub-types was not the source of heterogeneity. But sensitivity analysis showed that the results of first anal exhaust were robust (Figure S4).
Findings—postoperative complications
Several different adverse events and toxicities were reported (data shown in Table 6). In the meta-analysis, patients treated with either ERAS or conventional care from ten studies were included for analysis of postoperative complications. ERAS group had significant lower incidence of nausea than the conventional care group (overall RR =0.62, 95% CI: 0.40, 0.94, P=0.024). However, there was no statistical difference of other postoperative complications listed in Table 7 between ERAS and conventional care arms (P>0.05).

Full table
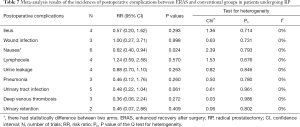
Full table
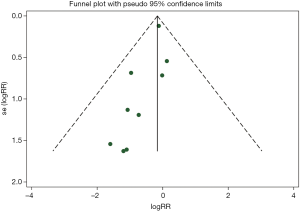
Publication bias
The shape of the funnel plot did not display any evidence of apparent asymmetry. Furthermore, the formal tests showed no substantial publication bias. The Egger’s test had a P value of 0.463 while the Begg’s test had a P value of 0.115 (Figure S5).
Discussion
In recent years, minimally invasive RP and open RP have been widely used for the management of Pca patients. Minimally invasive RP is advantageous because it minimizes operative injuries and reduces complications compared to open surgery. However, there seemed to be no significant difference in efficacy between these two sub-types of RP (4,28,29). In some countries, open RP is still an option for Pca patients. Currently, many medical centers have implemented the ERAS into RP programs. The ERAS protocol is a standardized perioperative care pathway aimed at minimizing the stress of surgery, reduce postoperative morbidity, shorten hospital stay and accelerate recovery. ERAS has been widely implemented in abdominal and gynecologic surgery (7,30,31). Nonetheless, results from clinical trials are not compelling enough to support any definitive conclusions about the superiority of ERAS in RP programs. In this study, we performed a systematic review and meta-analysis on the efficacy and safety of ERAS and conventional care for Pca patients undergoing RP from 10 primary studies. Among the ten, five were RCTs, four were retrospective trials and one was a prospective cohort study. Results indicated that the ERAS group had significantly shorter hospital stay, shorter time to first defecation, shorter time to first anal exhaust, and lower incidence of nausea compared to the conventional group. However, there was no statistical difference in intraoperative outcomes, catheter stay and other postoperative complications between ERAS and conventional group.
Overall survival (OS) and recurrence-free survival (RFS) are the key outcomes for evaluating the efficacy of a surgery. However, the trials included in this meta-analysis did not assess the OS nor RFS, because they were all short-term studies. As such, long-term clinical trials are needed to compare the OS and RFS of these two groups. ERAS group had significantly shorter hospital stay compared to the conventional care group (P<0.001). However, the difference in intraoperative outcomes and catheter stay between ERAS and conventional care arms was not significant (P>0.05). Hospital stay is an important outcome for any surgery. Sugi 2017 reported that there was no significant difference in hospital stay between these two groups (P>0.05) (data shown in Tables 3,4) (20). Contrary to these findings, the ERAS group had a significantly shorter hospital stay period compared to the conventional group (P<0.05) in 8 trials used in this study (19,21-27). Similarly, Lin 2018 compared the hospitalization costs between these two groups and found out that the ERAS group could significantly reduce the hospitalization costs compared to the conventional care group (6.1 vs. 7.2 thousand USD, P<0.001) (22).
Meta-analysis results also revealed that the ERAS group had significantly shorter times to first defecation (P=0.008) and first anal exhaust compared to the conventional group (P=0.001). Time to first defecation and anal exhaust were two indicators of postoperative recovery of intestinal function. The ERAS protocol suggested omission of preoperative bowel preparation, preoperative carbohydrate loading, and restricted fluid therapy. Evidently, ERAS group had a better postoperative intestinal function recovery than the conventional group.
Another potential advantage of ERAS would be the reduction of the frequencies of postoperative complications. Huang 2018 reported that ERAS could significantly reduce the incidence of pain compared to the conventional group (data shown in Tables 3,4, P=0.004) (21). In the same line, Okamura 2013 reported that there was significantly lower incidence of fever above 38 °C in ERAS group compared to the conventional group (1.9% vs. 3.5%, P=0.014) (23). Similarly, Gralla 2007 reported that there was significantly lower incidence of penoscrotal complications in the ERAS group compared to the conventional group (5 vs. 12, P=0.04) (18). However, trials included for this study were not enough to accurately assess these complications between the two groups. As such, no definite conclusion could be drawn. Nonetheless, pooled meta-analysis results revealed that the ERAS group had significant lower incidence of nausea compared to the conventional care group. However, there were no significant differences in other postoperative complications between ERAS and conventional groups (P>0.05) (data shown in Table 7).
Heterogeneity is an important aspect in meta-analysis. In this study, statistical analysis revealed that heterogeneity was present in most aspects. Subgroup analysis performed indicated that the difference in surgery sub-types was not the source of heterogeneity. Although the sources of heterogeneity were not found, sensitivity analysis results indicated that the overall significance of the pooled estimates were not affected by any trial included in the study. Similarly, Begg’s and Egger’s tests were used to detect any publication bias that would introduce false positives in meta-analysis (17). No evidence of publication bias was detected. These results indicated that all the conclusions of this study were credible and verifiable.
However, this study is limited by several factors. The number of studies used was low because of lack of enough high-quality RCTs. As such, some complications could not be accurately assessed. Further to this, the trials included in this study were short-term studies, had inconsistent ERAS protocols and were open labelled. All these were factors that could have affected the outcomes of the study. In future studies, more rigorous long-term experiments needed to be designed to enable precise meta-analysis of all aspects between the ERAS and conventional care groups.
Conclusions
Evidently, the ERAS group had significantly shorter hospital stay, shorter time to first defecation, shorter time to first anal exhaust and lower incidence of nausea compared to the conventional care group. Both groups had similar incidences of other postoperative complications. Based on the consistence of the data presented so far in this and previous studies, ERAS has the potential to be used as a standard of care for Pca patients undergoing RP.
Acknowledgments
We thank our collaborators who helped with this study.
Funding: This work was supported by Joint Fund of Zhejiang Provincial Natural Science Foundation, China (Grant number LYY18H310002), Funding of Zhejiang Pharmaceutical Association (Grant number ZYYZL01) and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission, China (Grant number 2017KY345).
Footnote
Conflicts of Interest:All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.04.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Ilic D, Evans SM, Allan CA, et al. Laparoscopic and robot-assisted vs open radical prostatectomy for the treatment of localized prostate cancer: a Cochrane systematic review. BJU Int 2018;121:845-53. [Crossref] [PubMed]
- Allan C, Ilic D. Laparoscopic versus Robotic-Assisted Radical Prostatectomy for the Treatment of Localised Prostate Cancer: A Systematic Review. Urol Int 2016;96:373-8. [Crossref] [PubMed]
- Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 2009;55:1037-63. [Crossref] [PubMed]
- Bekelman JE, Rumble RB, Chen RC, et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol 2018;36:3251-8. [Crossref] [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Li S, Zhou K, Che G, et al. Enhanced recovery programs in lung cancer surgery: systematic review and meta-analysis of randomized controlled trials. Cancer Manag Res 2017;9:657-70. [Crossref] [PubMed]
- Pedziwiatr M, Mavrikis J, Witowski J, et al. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med Oncol 2018;35:95. [Crossref] [PubMed]
- Lemanu DP, Singh PP, Stowers MD, et al. A systematic review to assess cost effectiveness of enhanced recovery after surgery programmes in colorectal surgery. Colorectal Dis 2014;16:338-46. [Crossref] [PubMed]
- Li Z, Wang Q, Li B, et al. Influence of enhanced recovery after surgery programs on laparoscopy-assisted gastrectomy for gastric cancer: a systematic review and meta-analysis of randomized control trials. World J Surg Oncol 2017;15:207. [Crossref] [PubMed]
- Offodile AC 2nd, Gu C, Boukovalas S, et al. Enhanced recovery after surgery (ERAS) pathways in breast reconstruction: systematic review and meta-analysis of the literature. Breast Cancer Res Treat 2019;173:65-77. [Crossref] [PubMed]
- Azhar RA, Bochner B, Catto J, et al. Enhanced Recovery after Urological Surgery: A Contemporary Systematic Review of Outcomes, Key Elements, and Research Needs. Eur Urol 2016;70:176-87. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Gralla O, Haas F, Knoll N, et al. Fast-track surgery in laparoscopic radical prostatectomy: basic principles. World J Urol 2007;25:185-91. [Crossref] [PubMed]
- Magheli A, Knoll N, Lein M, et al. Impact of fast-track postoperative care on intestinal function, pain, and length of hospital stay after laparoscopic radical prostatectomy. J Endourol 2011;25:1143-7. [Crossref] [PubMed]
- Sugi M, Matsuda T, Yoshida T, et al. Introduction of an Enhanced Recovery after Surgery Protocol for Robot-Assisted Laparoscopic Radical Prostatectomy. Urol Int 2017;99:194-200. [Crossref] [PubMed]
- Huang Z, Yi L, Zhong Z, et al. Comparison of Fast-Track Versus Conventional Surgery Protocol for Patients Undergoing Robot-Assisted Laparoscopic Radical Prostatectomy: A Chinese Experience. Sci Rep 2018;8:8017. [Crossref] [PubMed]
- Lin C, Wan F, Lu Y, et al. Enhanced recovery after surgery protocol for prostate cancer patients undergoing laparoscopic radical prostatectomy. J Int Med Res 2019;47:114-21. [Crossref] [PubMed]
- Okamura K, Nojiri Y, Tanaka Y, et al. Changes in perioperative management of radical prostatectomy using clinical pathways according to a standardized care plan: a multi-institutional study. Int J Urol 2013;20:337-43. [Crossref] [PubMed]
- Abou-Haidar H, Abourbih S, Braganza D, et al. Enhanced recovery pathway for radical prostatectomy: Implementation and evaluation in a universal healthcare system. Can Urol Assoc J 2014;8:418-23. [Crossref] [PubMed]
- Dong N, Chan Y, Jia L, et al. Effect of rapid rehabilitation concept on postoperative treatment effect, compliance of pelvic floor muscle rehabilitation and urinary function of patients with prostate cancer. Oncol Progress 2018;16:1933-36.
- Yu H, Wang J. ERAS in Multidisciplinary Cooperation in Patients with Robot-assisted Laparoscopic. J Qilu Nursing 2018;24:18-21.
- Zhao B, Shi L, Yuan Y, et al. Application of FTS concept-guided measures in perioperative care in laparoscopic radical prostatectomy. Chin J Clin Oncol Rehabil 2018;25:717-20.
- Sujenthiran A, Nossiter J, Parry M, et al. National cohort study comparing severe medium-term urinary complications after robot-assisted vs laparoscopic vs retropubic open radical prostatectomy. BJU Int 2018;121:445-52. [Crossref] [PubMed]
- Ilic D, Evans SM, Allan CA, et al. Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst Rev 2017;9:CD009625. [PubMed]
- Li L, Chen J, Liu Z, et al. Enhanced recovery program versus traditional care after hepatectomy: A meta-analysis. Medicine (Baltimore) 2017;96:e8052. [Crossref] [PubMed]
- Trowbridge ER, Dreisbach CN, Sarosiek BM, et al. Review of enhanced recovery programs in benign gynecologic surgery. Int Urogynecol J 2018;29:3-11. [Crossref] [PubMed]