Early enteral feeding on esophageal cancer patients after esophageal resection and reconstruction
Introduction
Esophageal cancer patients are usually accompanied with malnutrition due to dysphagia attributable to esophageal stenosis, their underlying nutritional habits, or the systemic effects of the neoplasm (1-3). It has been well documented that the degree of malnutrition is positively correlated with the incidence of postoperative complications due to malnutrition-related depression of humoral and cellular immune function, changes in the inflammatory response, and delays or failures of the wound healing process (4-6). In other words, patients requiring selective surgery for esophageal neoplastic diseases often present a high incidence of serious complications. Infection, the most often occurring complication during the early postoperative period could even extend hospitalization.
Even though esophagectomy is the mainstay of curative treatment for esophageal cancer, total resection of the esophagus is always associated with postoperative catabolism, and changes in the metabolic, endocrine, neuroendocrine, and immune systems that contribute to high postoperative morbidity rates (7,8). The American Society of Parenteral and Enteral Nutrition recommends preoperative nutritional therapy in malnourished patients undergoing major gastrointestinal surgery (9,10). However, the inherent catabolic situation in esophageal patients does not usually allow preoperative treatment of malnutrition. For this reason, it is vital to provide adequate postoperative nutrition as soon as possible to counteract catabolism and reduce the severity of complications.
Over the past three decades, experimental and clinical studies have been performed to identify the optimal form of postoperative nutritional support and means of delivery. There is increasing evidence showing that the small bowel recovers its ability to absorb nutrients almost immediately after surgery, even in the absence of peristalsis. Early enteral feeding has also been shown to preserve the integrity of gut mucosa and its immunological function (11). However, current concerns regarding early postoperative feeding in esophageal patients may lead to high incidences of anastomotic leakage. This procedure’s high postoperative mortality rate has prevented physicians from attempting early postoperative oral diet after esophagectomy.
However, more and more studies have provided strong evidence that anastomotic leaks are growing rarer in esophagectomy, which can be attributed to the use of advanced medical techniques (12). It suggests that it may be feasible to start oral feeding early after esophagectomy. The aim of this study was to investigate the impact of early oral feeding on the postoperative course following esophagectomy in patients with esophageal carcinoma.
Methods
Patient recruitment
Inclusion criteria: (I) between 18 and 70 years old; (II) preoperative diagnosis of esophageal cancer in the middle or lower part of the esophagus and underwent minimally invasive Ivor-Lewis surgery; (III) willingness to sign an informed consent form. Patient exclusion criteria: (I) contradictions of enteral nutrition; (II) serum creatinine levels 2 times greater than the maximal limit of the normal range; (III) aspartate aminotransferase levels 3 times the maximum limit of the normal range or severe cholestasis or conjugated bilirubin levels 2 times the maximum limit of the normal range; (IV) severe cardiopulmonary dysfunction; (V) recent preoperative radiotherapy or chemotherapy; (VI) lack of provision of a written informed consent form or non-compliance with the study protocol; (VII) other undesirable trait. Exit criteria: (I) intolerant to nose-gut tubing during surgery; (II) refusal to replace nose-gut tubing after it came off; (III) failure to complete the treatment plan due to severe diarrhea or bloating; (IV) voluntary termination of treatment; (V) pathological confirmation that the patient’s condition was not esophageal cancer. This study was approved by ethics committee of Shenzhen People’s Hospital.
Patient management
Surgery
The recruited patients were randomly enrolled in either the early oral feeding (EOF) group or the simple tube feeding (STF) group. Esophagectomy and reconstruction were performed via minimally invasive Ivor-Lewis surgery, after the proximal stomach was freed under laparoscope for stomach tube formation and pyloroplasty, the thoracic esophagus was freed under thoracoscope, the surgical incision was extended to 7 cm for hand-assisted mechanical gastroesophageal anastomosis on top of cupula pleurae. The esophagoenteral anastomosis was embedded using standard procedures. Flocare® double lumen decompression tubes and nasojejunal feeding tubes were inserted into patients in both groups (Nutricia Export BV, Amsterdam, Netherlands). The feeding tube was inserted into the duodenum or to the lowermost anastomosis. The decompression tube, facilitating evacuation of the interponate, was positioned well within the stomach or in the part of the bowel replacing the esophagus.
Both groups were subjected to the same postoperative management regimen meant to foster rapid recovery. It included vibration treatment of the chest to facilitate expectoration (3 times/day), airway atomization (3 times/day), early activity (in-bed activity on day 1 and off-bed activity from day 2), and effective coughing. Neither group was given parenteral nutrition (PN).
Postoperative feeding
The nutritional goal was 25 kcal/(kg·day) in both groups. The nutritional goal of the EOF group on the first four postoperative days was achieved through tube feeding of a polymeric, isotonic, fiber-mixed suspension of enteral nutrition (1 kcal/mL, Jevity®, Abbott Nutrition) and a daily oral feeding of 250 mL of 5% glucose in normal saline solution. The absence of leakage was confirmed by esophageal iodine angiography. Then, on the 4th day after the operation, the chest tubes, nasogastric tubes, and nasal intestinal tube were removed, and the patients were started on the oral liquid diet. The STF group was tube-fed with an enteral nutrition mixture for the first 7 days after the operation instead. Complete disconnection of the chest, nasogastric, and nasal intestinal tubes and administration of oral liquid diet were performed after confirmation of the absence of anastomotic leaks on the 7th postoperative day.
Complications and postoperative observations
Safety indicators, including postoperative complications such as infection of incision, anastomotic leakage, pneumonia, and mortality were recorded. Outcomes of effectiveness, including the oral feeding recovery period, incidence of thirst, bowel movement recovery period, and duration of the postoperative hospital stay were also analyzed. Biochemical indicators, including preoperative and postoperative (1st, 3rd, and 7th) serum albumin (ALB), prealbumin (PA), transferrin (TRF), and C-reactive protein (CRP) levels were evaluated.
Statistical analysis
The SPSS19.0 software package was used for data analysis. Quantitative data were reported as mean ± standard deviation (SD) or median. For non-normal distribution data, data were described as means and medians. For pre-and postoperative data, self-control was used for data evaluation. The student’s t test was used to assess statistical differences between two groups concerning ordinal variables, and the chi-square test was used for nominal variables. In the exploratory analysis, a P value of <0.05 was considered indicative of statistical significance.
Results
Demographic characteristics
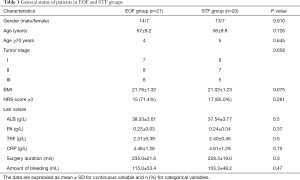
From Oct 2013 to Jan 2016, 23 patients were initially recruited in each group, while two cases in the EOF group and one case in the STF group were withdrawn from the study because of failure of the placement of duodenal tube during surgery. One more case in each group was also withdrawn postoperatively from the study because of bloating and diarrhea. Therefore, 41 consecutive patients (27 men and 14 women; mean age 58, range 18–70) with esophageal cancer (stages I–III), underwent minimally invasive Ivor-Lewis resection and reconstruction were recruited for the final analysis. There was no significant difference between the two groups regarding patients’ gender, age, tumor stage, or surgical methods (P>0.05) (Table 1).
Full table
Primary outcomes
Patients from the EOF group had significantly less postoperative recover time, shorter intervals since the first oral intake of semi-liquid diet and shorter postoperative hospital stays than those from STF group (P<0.05). EOF patients also showed the trend of shorter intervals until the first bowel movement, though difference was not statistically significant (P>0.05). The total oral feeding period in the STF group was longer than that of the EOF group. However, incidence of thirst was lower in the EOF group compared with STF group. The total post-operative hospitalization period was also shorter in the EOF group than that of the STF group (Table 2).

Full table
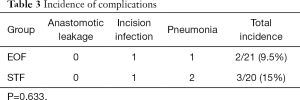
There were no peri-operative deaths or anastomotic leaks in either group. One case in each group suffered purulent in incision. Pneumonia was found in one case in the EOF group and two in the STF group, but the incidence of complications showed no significant differences between the two groups (Table 3).
Full table
Laboratory findings
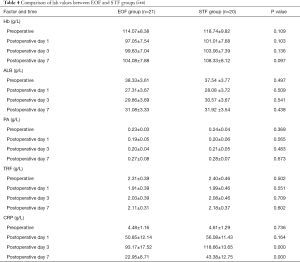
Intra-group comparison showed that all laboratory outcomes, including the levels of serum albumin, pre-albumin, and transferrin decreased at first but then gradually recovered. There were significant no differences of these biochemical indicators, between the EOF and STF groups (P>0.05).
C-reactive protein (CRP) was used as an indicator of inflammation. Comparison of both groups showed that CRP increased on the 1st, 3rd, and 7th postoperative days, and the highest level was found on the 3rd day and lowest on the 7th day. CRP levels on the 7th postoperative day of EOF group were significantly lower compared with that of STF group (P<0.05) (Table 4).
Discussion
Although it was usually considered that early feeding would lead to high incidence of anastomotic leakage, our study did not show peri-operative deaths or anastomotic leaks in either EOF or STF groups after esophagectomy and reconstruction of esophagus under thoracoscope. The recovery time, intervals since the first oral intake of semi-liquid diet, length of post-operative hospital stay in the EOF group were less than those of the STF group in our study. It was also found that early feeding seemed to reduce CRP of the patients than STF, which indicated that EOF might reduce acute inflammatory reaction after surgery.
Malnutrition, cancer and surgical trauma can impair the host’s defense and recovery and thus increasing the risk of postoperative complications such as infection and anastomotic issues. They can even lead to death (13,14). Patients with esophageal carcinoma are particularly easy to accompany with postoperative complications because malnutrition occurs commonly. Whether early feeding is benefit or not is still on controversy. The results of our study in accordance with a previous study. Of the patients accepting early feeding after esophagectomy, there were no significant differences of length of stay or anastomotic complications compared with routing feeding groups (15). For patients with jejunostomy, the mortality rate was found to be higher, however, it was not correlated to feeding ways. Furthermore, data from a systematic review also supported our results that early oral feeding was associated with a reduced length of stay with delayed oral feeding, without increased complication rates (16).
Enteral nutrition has been well recognized as an economical, safe, and effective nutritional support method that complies with the physiological state, helps to maintain the digestive tract morphology and function, operates in a simple way, and has few complications (17). In addition to these advantages, enteral nutrition during and after certain surgical insults has other benefits including inhibition of energy expenditure, the cytokine response, the secretion of stress hormones, and bacterial translocation (18-21).
Current peri-operative nutritional support for patients after esophagectomy is commonly achieved through total parenteral nutrition combined parenteral with enteral nutrition, or enteral nutrition alone. In clinical practice and with the advances in the role of the gastrointestinal tract in host defense, early enteral feeding after abdominal surgery has been favored over parenteral feeding (22,23). The primary goal of nutritional care has also changed from the provision of enough calories to cover a patient’s needs to the restoration of optimal metabolic and immune responses (7). As a consequence, sequential postoperative enteral nutrition, e.g., nutrition tube (jejunum fistula or naso-jejunum tube) feeding within 48 hours after surgery combined with oral fasting, then replaced with fluid diet on the 7–10th postoperative day, is the currently the standard nutrition regimen recommended by guidelines (24,25). Although early sequential postoperative enteral nutrition maintains postoperative bowel function and great progress in total parenteral nutrition, nasojejunal feeding tubes carry some risk of nasopharynx and intestinal mucosal injury and aspiration pneumonia, which occur in about 7% of patients (26). We did not observe any case of aspiration in this study, since evacuation of the stomach, the jejunum or the colon was routinely done in both groups, whereas the use of a double lumen decompression tube facilitated enteral feeding, proximal evacuation notwithstanding.
There were some evidences indicating that esophageal carcinoma was correlated with inflammation. In a retrospective study with 423 cases who were diagnosed with esophageal squamous cell carcinoma, value of CRP/albumin was used to evaluate the prognosis, which was suggested to be a promising inflammation-based prognostic score (27). Combination use of C-reactive protein (CRP) and carcinoembryonic antigen (CEA) was also an independent prognostic factor in patients with esophageal squamous cell carcinoma (28). Furthermore, CRP could be used to evaluate the prognosis of patients to be treated with surgery (29). Immuno-nutrition was confirmed to be helpful to attenuate inflammation for esophageal cancer patients (30). In our study, we found that early feeding could reduce CRP, which be due to two reasons. First, anti-inflammatory compounds were deposited in the nutritional fluids. Second, early feeding may promote the recovery of the patients. The anti-inflammatory effect of early feeding should be confirmed by further studies, which would be one of the important reasons for improving the clinical prognosis, and shortening the hospital stays.
In conclusion, the early oral feeding approach significantly reduced the time until postoperative ambulation, interval until the first semi-liquid food intake, time of hospital stay, and CRP levels in patients who underwent esophagectomy for esophageal carcinoma. Mortality and complications did not differ significantly. This study indicates that the EOF approach is safe and effective method in the management of esophagectomy patients.
Acknowledgments
Funding: Financial support for this work was provided by the Natural Science Foundation of China (grants 31500727), Science and Technology Project of Shenzhen (JCYJ20150402111430624), Shenzhen Municipal Health and Family Planning Commission (201501030) and Chen Jingyu Team of Sanming Project of Medicine in Shenzhen (SZSM201812058).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2020.04.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Shenzhen People’s Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Okamoto Y, Okano K, Izuishi K, et al. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg 2009;33:1815-21. [Crossref] [PubMed]
- Chang HR, Bistrian B. The role of cytokines in the catabolic consequences of infection and injury. JPEN J Parenter Enteral Nutr 1998;22:156-66. [Crossref] [PubMed]
- Bistrian BR, Blackburn GL, Hallowell E, et al. Protein status of general surgical patients. JAMA 1974;230:858-60. [Crossref] [PubMed]
- Nixon DW, Heymsfield SB, Cohen AE, et al. Protein-calorie undernutrition in hospitalized cancer patients. Am J Med 1980;68:683-90. [Crossref] [PubMed]
- Meakins JL. Host defense mechanisms in surgical patients: effect of surgery and trauma. Acta Chir Scand Suppl 1989;550:43-51; discussion 51-3. [PubMed]
- Decker D, Schondorf M, Bidlingmaier F, et al. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996;119:316-25. [Crossref] [PubMed]
- Fujitani K, Tsujinaka T, Fujita J, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg 2012;99:621-9. [Crossref] [PubMed]
- Yu G, Chen G, Huang B, et al. Effect of early enteral nutrition on postoperative nutritional status and immune function in elderly patients with esophageal cancer or cardiac cancer. Chin J Cancer Res 2013;25:299-305. [PubMed]
- Huhmann MB, August DA. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) Clinical Guidelines for Nutrition Support in Cancer Patients: nutrition screening and assessment. Nutr Clin Pract 2008;23:182-8. [Crossref] [PubMed]
- McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33:277-316. [Crossref] [PubMed]
- Sand J, Luostarinen M, Matikainen M. Enteral or parenteral feeding after total gastrectomy: prospective randomised pilot study. Eur J Surg 1997;163:761-6. [PubMed]
- Tabatabai A, Hashemi M, Mohajeri G, Ahmadinejad M, Khan IA, Haghdani S. Incidence and risk factors predisposing anastomotic leak after transhiatal esophagectomy. Ann Thorac Med 2009;4:197-200. [Crossref] [PubMed]
- Lundy J, Lovett EJ 3rd, Wolinsky SM, et al. Immune impairment and metastatic tumor growth: the need for an immunorestorative drug as an adjunct to surgery. Cancer 1979;43:945-51. [Crossref] [PubMed]
- Xu J, Zhong Y, Jing D, et al. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg 2006;30:1284-9. [Crossref] [PubMed]
- Wheble GA, Benson RA, Khan OA. Is routine postoperative enteral feeding after oesophagectomy worthwhile?. Interact Cardiovasc Thorac Surg 2012;15:709-12. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr 2015;34:1-6. [Crossref] [PubMed]
- Heyland DK, Cook DJ, Guyatt GH. Enteral nutrition in the critically ill patient: a critical review of the evidence. Intensive Care Med 1993;19:435-42. [Crossref] [PubMed]
- Mochizuki H, Trocki O, Dominioni L, et al. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg 1984;200:297-310. [Crossref] [PubMed]
- Fong YM, Marano MA, Barber A, et al. Total parenteral nutrition and bowel rest modify the metabolic response to endotoxin in humans. Ann Surg 1989;210:449-56; discussion 456-7. [Crossref] [PubMed]
- Saito H, Trocki O, Alexander JW, et al. The effect of route of nutrient administration on the nutritional state, catabolic hormone secretion, and gut mucosal integrity after burn injury. JPEN J Parenter Enteral Nutr 1987;11:1-7. [Crossref] [PubMed]
- Lin MT, Saito H, Fukushima R, et al. Route of nutritional supply influences local, systemic, and remote organ responses to intraperitoneal bacterial challenge. Ann Surg 1996;223:84-93. [Crossref] [PubMed]
- Kudsk KA, Tolley EA, DeWitt RC, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enteral Nutr 2003;27:1-9. [Crossref] [PubMed]
- Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 1992;216:172-83. [Crossref] [PubMed]
- American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. Clinical Guidelines for the Use of Parenteral and Enteral Nutrition in Adult and Pediatric Patients, 2009. JPEN J Parenter Enteral Nutr 2009;33:255-9. [Crossref] [PubMed]
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) (published correction appears in JPEN J Parenter Enteral Nutr. 2016 Nov;40(8):1200). JPEN J Parenter Enteral Nutr 2016;40:159-211. [Crossref] [PubMed]
- Baeten C, Hoefnagels J. Feeding via nasogastric tube or percutaneous endoscopic gastrostomy. A comparison. Scand J Gastroenterol Suppl 1992;194:95-8. [Crossref] [PubMed]
- Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 2015;15:350. [Crossref] [PubMed]
- Huang Y, Liu JS, Feng JF. The combination of preoperative serum C-reactive protein and carcinoembryonic antigen is a useful prognostic factor in patients with esophageal squamous cell carcinoma: a combined ROC analysis. Onco Targets Ther 2015;8:795-803. [Crossref] [PubMed]
- Song ZB, Lin BC, Li B, et al. Preoperative elevation of serum C-reactive protein as an indicator of poor prognosis for early-stage esophageal squamous cell carcinoma. Kaohsiung J Med Sci 2013;29:662-6. [Crossref] [PubMed]
- Sunpaweravong S, Puttawibul P, Ruangsin S, et al. Randomized study of antiinflammatory and immune-modulatory effects of enteral immunonutrition during concurrent chemoradiotherapy for esophageal cancer. Nutr Cancer 2014;66:1-5. [Crossref] [PubMed]