
Antipsychotics-associated obsessive-compulsive symptoms: individualized treatments and clinical benefits of memantine: a case report
Introduction
Obsessive-compulsive symptoms (OCS) or obsessive-compulsive disorder (OCD) are more pronounced in chronic or late stage schizophrenia, and the drug-associated factor is an important aspect of the pathological mechanism. Despite the fact that some second generation antipsychotics (SGAs) are also used as synergists for refractory OCD, there were a large number of case reports of SGAs inducing and deteriorating OCS, as SGAs have replaced the first generation antipsychotics (FGAs) to become the mainstream drug in the treatment of schizophrenia. When schizophrenia is accompanied by SGAs-associated OCS, treatment often faces a dilemma of either aggravated OCS or poorly controlled schizophrenia. Consequently, this state could cause immense pain to patients, significantly reduce drug compliance, and eventually worsen the clinical prognosis.
We here report a patient who experienced intractable SGAs-associated OCS which were refractory to SSRI, finally acquired relatively satisfied state by changing antipsychotics and an adjunctive therapy with memantine. The significance of the case report is mainly reflected in three aspects. First, though memantine is the adjunctive agent most consistently showing an effective impact in primary OCD, this is the first case to report its benefit in SGAs-associated OCS. Second, in this case, the process of switching antipsychotics which caused corresponding changes in the severity of OCS is an interesting and seldom-reported phenomenon, had once again highlighted the role of the 5-HT2 receptor blockade mechanism in SGAs-associated OCS. Third, it has not been reported that SGAs-associated OCS took place in a background of a related social-psychological factor. We present the following case in accordance with the CARE Guideline.
Case presentation
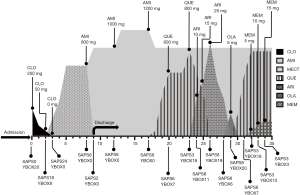
A 34-year-old man met the ICD-10 criteria for schizophrenia at the age of 17, with initial manifestations of severe auditory hallucination, delusions of reference and persecution. During his first admission in our hospital 17 year ago, risperidone was the first antipsychotic to be used. However, auditory hallucinations remained evident after 1 months of treatment at doses of 6 mg/day. Therefore, risperidone was withdrawn later and clozapine was started and titrated up to 250 mg/day. With this treatment, his psychotic symptoms disappeared gradually. Though he stayed most of the time at home, he was able to take care of himself and join some social gatherings. He had no history of OCD or personality disorder (OCPD).
About 4 year ago, the patient had a urinary tract infection after making a sexual intercourse with a local sex worker. His infection soon recovered under the doctor’s treatment, but he believed that whoring behavior is immoral, and sank into self-reproach and felt miserable from then on. Soon after, a clearly visible image of Chinese character ‘scum’ (means: the dregs of society) recurred in his mind intrusively and unwantedly, which made him feel ego dystonic. In the following 2 years, paroxetine (30 mg/day), escitalopram (20 mg/day), sertraline (150 mg/day), and clomipramine (150 mg/day) were successively prescribed by the local outpatient doctors, but besides clomipramine which caused his dysuria partially alleviated his obsessions, all the others did not work. And unfortunately, these medications even exacerbated his psychosis, for he developed new-onset delusion of persecution against his father. Yet, with the discontinuation of these medications because of their poor response and bad tolerance, his new-onset delusion of persecution fade away.
One year ago, he requested for hospitalized treatment on his own will because of his marked distress. Electroencephalography (EEG) and brain computed tomography (CT) scan were unremarkable. The rapid plasma regain test (RPR), treponema pallidum particle agglutination test (TPPA) and anti-HIV antibody test were all negative. We first reduced the dosage of clozapine with the consideration of the fact that clozapine could yield full threshold OCD or incur an exacerbation of pre-existing OCS. As expected, his obsessive image became less frequent and intense when the dose of clozapine was tapered to 50 mg/day. For the moment, however, his psychotic symptoms relapsed which presented as auditory hallucination and delusions of reference. Even so, clozapine was eventually withdrawn, and his refractory obsessive image finally fade away. The total Yale-Brown Obsessive-Compulsive Scale (YBOCS) score decreased from 20 to 0. By this time, amisulpride which virtually absent from blocking the 5-HT2 receptor, together with modified electric convulsive treatment (MECT) were used to control schizophrenia. Amisulpride was finally increased to 1,000 mg/day in the following 6 weeks. With the treatment, his psychotic symptoms almost fully resolved without causing any OCS.
The patient remained stable within about 1 month after discharging from the hospital. However, his idea of reference came back later without obvious incentive, resulted in anguished experience and impaired socialization. The dosage of amisulpride was increased to 1,200 mg/day in outpatient clinic. However, after 4 weeks of treatment, his psychiatric symptoms had not been eased and obvious static tremor was observed. Therefore, amisulpride was decreased back to 1,000 mg/day and quetiapine was added, beginning with 50 mg/day and progressively increased to 600 mg/day. Under this dosage, his idea of reference was partially alleviated, however, his obsessive image recurred, though the recurred obsession was still in his tolerable range. Since he still asked for further improvement of the idea of reference for a better personal relationship, quetiapine 800 mg/day was tried. Unfortunately, while increased quetiapine gained a satisfied improvement of the idea of reference, he again experienced recurrently insufferable obsessive image as before. Thereafter, aripiprazole or olanzapine as an alternate of quetiapine was also been used, but both they did not better improve his idea of reference. Besides, both of them worsened the OCS to some extent, especially for olanzapine, with even low doses of olanzapine (5 mg) had prompted a rapid exacerbation of his OCS. Thereby, therapy regimen was adjusted back to amisulpride 1,000 mg/day and quetiapine 800 mg/day. Memantine was prescribed after signing an informed consent regarding its off-label use, the treatment started with 5 mg/day, titrated to 15 mg/day in week 3. After 3 weeks of treatment, an evident improvement was seen in his OCS, his YBOCS score had fallen from 18 to 3 (for details of the course see Figure 1). The treatment regimen was maintained, and his condition was stable in the following 6 months’ follow-up.
Discussion
Schizophrenia with OCS can mainly be classified into three clinical phenotypes: OCS are part of active psychotic illness, in other words, schizophrenia occurs with obsessive-compulsive features; schizophrenia comorbid with primary OCD; de novo OCS associated with antipsychotic therapy (1). Furthermore, there are two subtypes of antipsychotics-associated OCS: new-onset OCS and aggravated existing OCS. Among SGAs, clozapine, olanzapine, and risperidone have the most significant relationship with antipsychotic-associated OCS. Quetiapine, aripiprazole and ziprasidone have also been reported to cause antipsychotic-associated OCS, and clozapine has the highest risk of OCS that new onset of OCS could reach as high as 20–28% and the incidence of aggravated existing OCS is 10–18%, clozapine could make OCS which are considered as a clinically sub-threshold state reach to full threshold OCD, in some cases (2).
It is well known that the pathological mechanism of OCD is the result of complex interaction of neurobiology, genetics and environment. Historically, dysfunction of the serotonin system is considered to be a major factor given the selective response to serotonergic drugs (3).Although the mechanism of SGAs-associated OCS are not entirely definite, it is believed that the central 5-HT dysfunction is most closely related to its pathophysiology (2).This theory has received support in many ways. Firstly, Ma et al. found that the serum 5-HT levels in clozapine-induced blockade of the 5-HT system were similar to those in OCD (4). Secondly, SGAs-induced OCS can be alleviated by SSRI (5). Thirdly, in schizophrenic patients, 70% of SGAs such as clozapine, olanzapine and risperidone that cause OCS have strong 5-HT blocking effects (1). SGAs (such as clozapine or olanzapine) with a strong 5-HT blocking effect result in a higher severity of and incidence of OCS than SGAs (such as aripiprazole) with a strong DA blocking effect (6). Moreover, FGAs usually do not aggravate or worsen OCS, and can even improve OCS (7). Similarly, the use of SGAs (such as amisulpride) which mainly block DA receptors could acquire these benefits (8). Amisulpride is a kind of SGAs, however, its pharmacological action is different from that of the others. It has an extremely weak blocking effect to 5HT2 receptors and its antipsychotic effect primarily depends on the highly selective blockade of the D2/D3 receptors. Therefore, it can be assumed that amisulpride should not cause or worsen OCS in schizophrenic patients. Indeed, a few cases about SGAs induced OCS alleviated by changing to amisulpride had been reported (8,9), resembling to ours.
In this present case, the use of SGAs may outweighs the primary factor in the genesis of his OCS. Firstly, the patient had no history of OCD or OCPD, and the OCS occurred in the remission phase of schizophrenia. Secondly, his OCS occurred during the use of clozapine, and obtained marked alleviation after dose-reduction, and once completely vanished with the discontinuation of clozapine, but recurred with the re-use of SGAs (quetiapine, aripiprazole, olanzapine) which also had a strong blocking effect on 5-HT2 receptor. Thirdly, OCS were not observed during the monotherapy of amisulpride which barely blocks 5-HT2 receptor. Therefore, we thus have reasons to speculate that SGAs with a high affinity 5-HT2 receptor had played the triggering and accelerating roles in his OCS, and even made his OCS reach to full threshold of OCD, though the occurrence of his OCS had a psychogenic origin (a self-blamed sexual behavior).
Current managements of SGAs-caused OCS are still as follows: the dose of antipsychotics should be reduced first, and other antipsychotics such as amisulpride or haloperidol should be used instead if it is ineffective. SSRI is added to the treatment when the antipsychotics do not work, and another SSRI could be used when the previous one does not work (1). Unfortunately, in the present case, amisulpride replacement therapy was insufficient to achieve full remission of his delusion. It also appeared that SSRI did not confer any advantage to his OCS in the tolerable range. Since it was hard to find a balance point around the dopaminergic and serotoninergic system, our attention had shifted towards to other neurotransmitter systems.
So far, the studies of the pathogenesis of OCD are largely focused on the cortical-striatal-thalamocortical (CSTC) circuitry, and the orbitofrontal cortex (OFC) was regarded as a critical brain area of this circuit. According to the prevailing theoretical model, the CSTC circuit involves direct and indirect pathways which work in balance. The direct pathway plays a role of self-reinforcing positive feedback loop, so as to initiate and continuate behaviors, versus the indirect pathway which functions as a negative feedback loop to inhibit behaviors and switch between behaviors (10). Hyperactivity of the direct pathway, or hypoactivity of the indirect pathway was thought to produce OC symptoms (11). As the CSTC’s direct pathway is glutamatergic, glutamatergic synaptic dysfunction within this area could probably be implicated in the pathogenesis of OCD.
Indeed, the pathophysiology studies of OCD have revealed the close correlation with glutamate on different levels. For instance, imaging studies using proton magnetic resonance spectroscopy have shown the glutamate hyperactivity in the OFC in patients with OCD (12). Interestingly, in research of pathology on SGAs-induced OCS in schizophrenia, a stronger brain activation was observed by functional magnetic resonance imaging in the OFC during a response inhibition task in patients taking SGAs with a prominent anti-serotoninergic profile compared to patients receiving SGAs with a prominent dopaminergic blockade, correlated with more severe OCS. A correlation was also observed between OFC activation and clinical severity of obsessions reflecting in the YBOCS (13). In primary OCD, genetic researchers have found significant associations with glutamate related genes including glutamate transporter (SLC1A1) gene (14) which has also been implicated in SGAs-induced OCS. Results suggested that SLC1A1 rs2228622 interacted with DLGAP3 (disks large associated protein 3) rs7525948 to significantly predict SGA-emergent OCS (15). SLC1A1 variants were found to increase risk and severity of OCS among schizophrenia patients treated with clozapine (16). In Korean schizophrenic patients, a significant association was found with the sequence variations in SLC1A1and the susceptibility to SGA-induced OCS (17). It is worth noting that, in this case, the 5-HT2 receptor blockade effect in the genesis of his OCS and the effectiveness of memantine in the treatment of his OCS may suggest interactions between serotonin and glutamate pathways in the pathology of SGAs-associated OCS.
There are growing evidences from clinical research on the benefits of the glutamate receptor antagonists in treating of pharmacoresistant OCD. As an adjunctive therapy, memantine is the agent most consistently showing a favorable impact in primary OCD. Besides its de-excitation effect on direct pathway of the CSTC circuit, another possible mechanisms including a targeted de-excitation effect in the temporal lobes and connected brain regions, were thought to contribute to a further alleviating of OCD symptoms (14). In schizophrenia patients, memantine has also been explored to treat of negative and cognitive symptoms as an augmentation. According to a recent Meta-analysis, memantine showed good performance in safety and tolerability among schizophrenia patients, for it was not associated with an increased risk of adverse drug events or of discontinuation due to adverse drug events (18). Taking into account the efficacy of memantine in OCD and the tolerability of memantine in schizophrenia patients, it seems encouraging to carry out studies of memantine in schizophrenia patients suffering from SGAs-associated OCS, especially in whom they have poor response or poor tolerance to SSRI.
Conclusions
In sum, in schizophrenics taking antipsychotic drugs with predominant anti-serotoninergic profiles, though OCS may sometimes occurs after psychological events, the exacerbating effect of drugs should not be neglected. Serotonergic blockade is still the mainstream hypothesis of the etiology of SGAs-associated OCS, which should be recognized by clinicians. Thus, adjusting to antipsychotics with predominant dopaminergic blockade and/or combining use of SSRI are still the mainstay approaches. However, it’s often hard to strike the right balance between the therapeutic action and the incidences of OCS brought by antipsychotics. For some individuals, it’s may also hard to find the proper balance between the OCS-improving effect and the psychosis-exacerbating effect or other intolerance brought by SSRI. In such cases, agents involve other neurotransmitter systems should be taken into consideration, and memantine is an approach worth considering. In addition, since SGAs-associated OCS are sometimes dose-dependent, we recommend prescribing the minimum effective dose and gradual titration. Patients administering SGAs should be routinely monitored for OCS throughout the course of treatment. A longer follow-up is needed.
Acknowledgments
Funding: Science and Technology Development Fund of Shanghai Pudong New Area (PKJ2018-Y27). Key Discipline Construction Project of Pudong Health Bureau of Shanghai (PWZxk2017-29). The Outstanding Clinical Discipline Project of Shanghai Pudong (PWYgy2018-10).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hwang MY, Kim SW, Yum SY, et al. Management of schizophrenia with obsessive-compulsive features. Psychiatr Clin North Am 2009;32:835-51. [Crossref] [PubMed]
- Fonseka TM, Richter MA, Muller DJ. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: a review of the experimental literature. Curr Psychiatry Rep 2014;16:510. [Crossref] [PubMed]
- Fenske JN, Petersen K. Obsessive-Compulsive Disorder: Diagnosis and Management. Am Fam Physician 2015;92:896-903. [PubMed]
- Ma N, Tan LW, Wang Q, et al. Lower levels of whole blood serotonin in obsessive-compulsive disorder and in schizophrenia with obsessive-compulsive symptoms. Psychiatry Res 2007;150:61-9. [Crossref] [PubMed]
- Scheltema Beduin AA, Swets M, Machielsen M, et al. Obsessive-compulsive symptoms in patients with schizophrenia: a naturalistic cross-sectional study comparing treatment with clozapine, olanzapine, risperidone, and no antipsychotics in 543 patients. J Clin Psychiatry 2012;73:1395-402. [Crossref] [PubMed]
- Schirmbeck F, Zink M. Clozapine-induced obsessive-compulsive symptoms in schizophrenia: a critical review. Curr Neuropharmacol 2012;10:88-95. [Crossref] [PubMed]
- McDougle CJ, Goodman WK, Leckman JF, et al. Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder. A double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 1994;51:302-8. [Crossref] [PubMed]
- Kim SW, Shin IS, Kim JM, et al. Amisulpride improves obsessive-compulsive symptoms in schizophrenia patients taking atypical antipsychotics: an open-label switch study. J Clin Psychopharmacol 2008;28:349-52. [Crossref] [PubMed]
- Kim SW, Shin IS, Kim JM, et al. The 5-HT2 receptor profiles of antipsychotics in the pathogenesis of obsessive-compulsive symptoms in schizophrenia. Clin Neuropharmacol 2009;32:224-6. [Crossref] [PubMed]
- van den Heuvel OA, van Wingen G, Soriano-Mas C, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol 2016;26:810-27. [Crossref] [PubMed]
- Wu K, Hanna GL, Rosenberg DR, et al. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav 2012;100:726-35. [Crossref] [PubMed]
- Nakao T, Okada K, Kanba S. Neurobiological model of obsessive-compulsive disorder: evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin Neurosci 2014;68:587-605. [Crossref] [PubMed]
- Schirmbeck F, Mier D, Esslinger C, et al. Increased orbitofrontal cortex activation associated with "pro-obsessive" antipsychotic treatment in patients with schizophrenia. J Psychiatry Neurosci 2015;40:89-99. [PubMed]
- Vlcek P, Polak J, Brunovsky M, et al. Role of Glutamatergic System in Obsessive-Compulsive Disorder with Possible Therapeutic Implications. Pharmacopsychiatry 2018;51:229-42. [Crossref] [PubMed]
- Ryu S, Oh S, Cho EY, et al. Interaction between genetic variants of DLGAP3 and SLC1A1 affecting the risk of atypical antipsychotics-induced obsessive-compulsive symptoms. Am J Med Genet B Neuropsychiatr Genet 2011;156B:949-59. [Crossref] [PubMed]
- Cai J, Zhang W, Yi Z, et al. Influence of polymorphisms in genes SLC1A1, GRIN2B, and GRIK2 on clozapine-induced obsessive-compulsive symptoms. Psychopharmacology (Berl) 2013;230:49-55. [Crossref] [PubMed]
- Kwon JS, Joo YH, Nam HJ, et al. Association of the glutamate transporter gene SLC1A1 with atypical antipsychotics-induced obsessive-compulsive symptoms. Arch Gen Psychiatry 2009;66:1233-41. [Crossref] [PubMed]
- Andrade C. Memantine as an Augmentation Treatment for Schizophrenia: Limitations of Meta-Analysis for Evidence-Based Evaluation of Research. J Clin Psychiatry 2017;78:e1307-9. [Crossref] [PubMed]


