
Effects of interlaminar epidural steroid injection in patients with moderate to severe lumbar central spinal stenosis: a prospective study
Introduction
Lumbar central spinal stenosis (LCSS) is defined as a narrowing of the spinal canal with encroachment on the neural structures by the herniation or bulging of intervertebral discs, osteophytes, and hypertrophy of the ligamentum flavum. LCSS is one of the most common causes of lumbar radiculopathy, which induces claudication and reduces functional ability and quality of life (1,2). Symptomatic LCSS is reported to be prevalent in about 27% of the general population (2).
For the management of the pain induced by LCSS, several treatment modalities, including medication, exercise, interventions, and surgery, have been applied (3-6). Interlaminar epidural steroid injection (ESI) has commonly been used in patients with LCSS (7-13). Corticosteroids inhibit the synthesis of various pro-inflammatory mediators (14). Accordingly, ESI can reduce nerve root inflammation induced by mechanical compression at the narrowed spinal canal. Several previous studies have demonstrated its safety and efficacy in alleviating pain due to LCSS (7-13). However, so far, little is known about the effects of ESI according to the severity of LCSS. Also, there has been no study that focused on the effectiveness of ESI in patients with moderate to severe LCSS.
Therefore, in the current study, we evaluated the outcome of interlaminar ESI in patients with chronic pain induced by moderate or severe LCSS, and compared the effects of interlaminar ESI between patients with moderate LCSS and patients with severe LCSS.
Methods
Patients
This was a single-center prospective observational study. This study was conducted in outpatient pain clinic in a university hospital. We prospectively evaluated 60 consecutive patients who had lower extremity pain due to LCSS according to the following inclusion criteria: (I) age between 20 and 79 years; (II) ≥6-month history of pain induced by LCSS, characterized by bilateral buttock and/or bilateral lower extremity pain in a diffuse distribution during walking or prolonged standing that is relieved by leaning forward or sitting; (III) pain intensity of at least 3 on a numeric rating scale (NRS; 0= no pain, 10= the worst pain); (IV) LCSS with moderate or severe degree on cross-sectional magnetic resonance imaging (MRI). Exclusion criteria were as follows: (I) the presence of foraminal stenosis, herniation of the lumbar disc, myelopathy, or infection on the spine; (II) previous history of spinal surgery, such as lumbar fusion or laminectomy; and (III) coagulation disorder. The Institutional Review Board of our hospital approved the study and all patients provided a signed informed consent form.
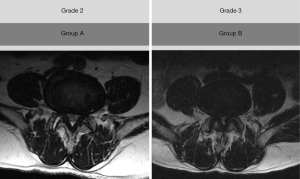
The severity of LCSS was assessed based on the findings of lumbar axial MRI according to Lee et al.’s study (14) (Figure 1). Grade 0 indicates no LCSS. Grade 1 refers to mild stenosis with clear separation of each cauda equina nerve root. Grade 2 means moderate stenosis with some cauda equina aggregation. Grade 3 is severe stenosis with the entire cauda equina as a bundle. Patients having LCSS with grade 2 or 3 were recruited to our study. We reclassified grade 2 as group A, in which the degree of LCSS was moderate. Grade 3 was reclassified as group B, in which the degree of LCSS was severe (Figure 1). Thirty patients were classified into group A, and the other 30 patients were classified into group B.
Interlaminar ESI
All injections were performed by a single interventional physiatrist who specialized in central spinal injections. Strict aseptic technique was used during all interlaminar ESI procedures. Patients were placed in prone position, and C-arm fluoroscopy (Siemens, Erlangen, Germany) was used for level identification and needle guidance. Lidocaine 1% was administered at the needle insertion site, and a 21-G Tuohy needle was inserted and placed into the epidural space via interlaminar approach, using the loss-of-resistance technique. The injection was performed at the level of LCSS. Adequate placement of the needle tip into the epidural space was confirmed with radiopaque contrast medium. After confirmation, 7.5 mg of dexamethasone with 4 mL of normal saline was injected.
Outcome measures
The assessments at pretreatment and follow-up periods were performed by one investigator; this investigator was blinded to the group allocation and did not participate in any treatments. Pain intensity was assessed using a numeric rating scale (NRS). The numeric rating scale is an 11-point scale (range score 0–10) for patient self-reporting of pain. The NRS scores were measured before treatment and at 1, 2, and 3 months after ESI. Successful treatment was defined as more than 50% reduction in the NRS score at 3 months when compared to the pretreatment NRS score. To validate the change in pain reduction, NRS scores were evaluated by assessing the difference between the pretreatment and the 3-month post-treatment scores [change in NRS (%) = [pretreatment score − score at three months after treatment]/pretreatment score ×100].
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS; v. 22.0, IBM Corporation, Armonk, NY). Demographic data and successful pain relief rate were compared between the two groups using the Mann-Whitney U test and chi-square test. Changes in NRS scores in all recruited patients as well as in the patients in each group were evaluated using repeated measure one-factor analysis. Repeated measure two-factor analysis was used to compare changes between groups over time. Multiple comparisons were obtained following a contrast using the Bonferroni correction. The level of statistical significance was set at P<0.05.
Results
Thirty patients in each group were included and received interlaminar ESI. Two patients in group B were lost to follow-up. Accordingly, 30 patients in group A and 28 patients in group B were followed for 3 months after interlaminar ESI. No adverse events were observed in either group. No significant intergroup differences were observed for demographic data (P>0.05; Table 1).

Full table
Overall, in all the recruited patients, the mean pain intensity decreased significantly over time (P<0.001; Figure 2A). The mean pain intensity was 4.6±1.3, 3.1±1.5, 3.4±1.6, and 3.6±1.5 before treatment and at 1, 2, and 3 months after treatment, respectively. Pain intensity was significantly lower at each evaluation time point compared to pretreatment values (P<0.001). Fourteen patients (24.1%) experienced successful pain relief (pain relief of ≥50%) after interlaminar ESI.
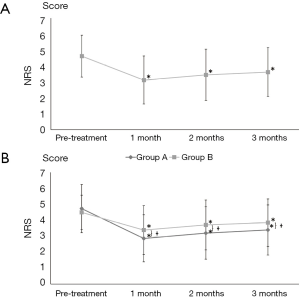
Intragroup analysis revealed that in group A, the mean pain intensity decreased from 4.7±1.5 before treatment to 2.9±1.5, 3.2±1.6, and 3.4±1.6 at 1, 2, and 3 months after treatment, respectively (Figure 2B). In group B, the mean pain intensity decreased from 4.5±1.1 before treatment to 3.4±1.5, 3.7±1.6, and 3.9±1.5 at 1, 2, and 3 months after treatment, respectively (Figure 2B). The mean pain intensity for each group was significantly different over time (P<0.001) (Figure 2B). In both groups, pain intensity was significantly lower at 1, 2, and 3 months compared to pretreatment values (P<0.001). Decreases in pain intensity over time were significantly larger in group A (P=0.025). In addition, decreases in pain intensity from pretreatment to each evaluation time point were significantly larger in group A compared to group B (1 month: P=0.027, 2 months: P=0.032, 3 months: P=0.029). Three months after treatment, 9 patients (30.0%) in group A and 5 patients (17.9%) in group B reported successful pain relief (pain relief of ≥50%). The rate of successful pain relief at 3 months after treatment was not significantly different between the two groups (P=0.109).
Discussion
In the current study, we evaluated the outcome of interlaminar ESI in patients with pain induced by moderate or severe LCSS. We also compared the effects of ESI between patients with moderate LCSS and patients with severe LCSS.
Although the symptoms of LCSS were significantly reduced after interlaminar ESI, the treatment was not sufficient to fully control pain in moderate or severe LCSS. About 25% of the patients in the current study reported successful pain relief (pain relief of ≥50%) at 3 months after treatment. In addition, the degree of pain relief after treatment was significantly lower in patients with severe LCSS than in patients with moderate LCSS. Only 18% of patients with severe LCSS showed successful response to interlaminar ESI, whereas 30% of patients with moderate LCSS showed successful pain relief.
In LCSS, narrowing of the spinal canal causes it to press against the spinal nerve roots, and also leads to compression of the vascular structures. Pinched nerve roots become inflamed and cause radicular pain (15). The compression of vascular structures produces arterial insufficiency and venous engorgement, leading to ischemic neuritis of nerve roots, which is correlated to neurological claudication (15). Corticosteroids injected into the epidural space can reduce inflammation in spinal nerve roots or tissues around the nerve roots via inhibition of various inflammatory mediators, such as cytokines, nitric oxide, lactate, phospholipase A2, proteoglycans, and immune response cells (16,17). Furthermore, decreased inflammation reduces edema, which can increase space in the lumbar spinal canal, thereby reducing the degree of compression in the spinal nerve roots and relieving arterial insufficiency and venous engorgement (15). In addition to an anti-inflammatory effect, corticosteroids inhibit neural transmission within the nociceptive C-fibers (17,18). These actions of corticosteroids are thought to reduce pain following ESI. However, in our patients, interlaminar ESI was not effective in most patients (75%), and the effect was less pronounced in patients with severe LCSS compared to patients with moderate LCSS. The effect of ESI in LCSS is still debatable. The poor response observed in our study is compatible with the results of some previous studies in which the pain associated with LCSS was not sufficiently reduced after ESI (12,19-21). We think that the increment of space in spinal canal and reduction of inflammation around the nerve roots after ESI might not have been sufficient to revert the moderate or severe narrowing of the spinal canal. In contrast, there have been several studies reporting a positive response of ESI on pain relief in patients with LCSS (7-11,13). However, the authors of those studies recruited patients with LCSS regardless of the degree of spinal stenosis, i.e., they recruited patients with mild, moderate, and severe LCSS. It is possible that the different treatment outcomes are due to the different degrees of stenosis included in each study.
Regarding the effect of ESI according to the severity of LCSS, to the best of our knowledge, only two studies have been reported (8,13). In 2014, Park et al. (8) reported that the grade of LCSS was not associated with the degree of pain relief after ESI. They graded LCSS using the same classification method as that used in our study. However, in their study, the number of patients with severe LCSS was only six, which was insufficient to arrive at a conclusion regarding the correlation between severity of LCSS and ESI. In 2015, Turner et al. (13) recruited 400 patients with LCSS, but they also reported that the severity of LCSS was not correlated with the treatment outcome after ESI. However, in their study, stenosis was classified subjectively into mild, moderate, and severe, and an objective measure was not used. Therefore, our study is the first to demonstrate the difference in treatment outcome after ESI according to the degree of LCSS based on objective classification methods. Moreover, our study is different from previous studies in that we recruited patients with moderate to severe LCSS, thus excluding those with mild LCSS.
In the current study, despite of only 24.1% of successful pain relief, pain intensity after interlaminar ESI was significantly decreased in patients with moderate to severe LCSS. Although the effect of interlaminar ESI in moderate to severe LCSS is relatively limited, it might be considerable option for alleviating pain. In addition, patients with severe LCSS showed worse treatment outcome, compared with those with moderate LCSS. The rate of successful pain relief in the patients with severe LCSS was 17.9%. In our opinion, this rate might be insufficient for application in patients with severe LCSS. Therefore, when treating patients with severe LCSS, clinicians should consider other techniques, such as bilateral transforaminal ESI (22), as alternatives; clinicians should also keep in mind the possibility of surgery. Before making a clear decision on the clinical applicability of interlaminar ESI for moderate to severe LCSS, some limitations of this study should be considered. First, our study is limited by its small sample size. Second, the functional outcome after ESI was not investigated. Further studies addressing these limitations are recommended.
Acknowledgments
Funding: The present study was supported by a National Research Foundation of Korea grant funded by the Korean government (grant no. NRF-2019R1F1A1061348).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Yeungnam University (No. 2019-05-044).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haig AJ, Tomkins CC. Diagnosis and management of lumbar spinal stenosis. JAMA 2010;303:71-2. [Crossref] [PubMed]
- Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J 2009;9:545-50. [Crossref] [PubMed]
- Chen E, Tong KB, Laouri M. Surgical treatment patterns among Medicare beneficiaries newly diagnosed with lumbar spinal stenosis. Spine J 2010;10:588-94. [Crossref] [PubMed]
- Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine (Phila Pa 1976) 2011;36:E1335-51. [Crossref] [PubMed]
- Shim E, Lee JW, Lee E, et al. Facet joint injection versus epidural steroid injection for lumbar spinal stenosis: intra-individual study. Clin Radiol 2017;72:96.e7-96.e14. [Crossref] [PubMed]
- Tosteson AN, Tosteson TD, Lurie JD, et al. Comparative effectiveness evidence from the spine patient outcomes research trial: surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine (Phila Pa 1976) 2011;36:2061-8. [Crossref] [PubMed]
- Friedly JL, Comstock BA, Turner JA, et al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med 2014;371:11-21. [Crossref] [PubMed]
- Park CH, Lee SH. Correlation between severity of lumbar spinal stenosis and lumbar epidural steroid injection. Pain Med 2014;15:556-61. [Crossref] [PubMed]
- Manchikanti L, Cash KA, McManus CD, et al. A randomized, double-blind controlled trial of lumbar interlaminar epidural injections in central spinal stenosis: 2-year follow-up. Pain Physician 2015;18:79-92. [PubMed]
- Manchikanti L, Cash KA, McManus CD, et al. Lumbar interlaminar epidural injections in central spinal stenosis: preliminary results of a randomized, double-blind, active control trial. Pain Physician 2012;15:51-63. [PubMed]
- Manchikanti L, Falco FJ, Pampati V, et al. Lumbar interlaminar epidural injections are superior to caudal epidural injections in managing lumbar central spinal stenosis. Pain Physician 2014;17:E691-702. [PubMed]
- Sivaganesan A, Chotai S, Parker SL, et al. Predictors of the efficacy of epidural steroid injections for structural lumbar degenerative pathology. Spine J 2016;16:928-34. [Crossref] [PubMed]
- Turner JA, Comstock BA, Standaert CJ, et al. Can patient characteristics predict benefit from epidural corticosteroid injections for lumbar spinal stenosis symptoms? Spine J 2015;15:2319-31. [Crossref] [PubMed]
- Lee GY, Lee JW, Choi HS, et al. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol 2011;40:1033-9. [Crossref] [PubMed]
- Akuthota V, Lento P, Soxa G. Pathogenesis of lumbar spinal stenosis pain: why does an asymptomatic stenotic patient flare? Phys Med Rehabil Clin N Am 2003;14:17-28. [Crossref] [PubMed]
- Greaves MW. Anti-inflammatory action of corticosteroids. Postgrad Med J 1976;52:631-3. [Crossref] [PubMed]
- Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand 1990;34:335-8. [Crossref] [PubMed]
- Li JY, Xie W, Strong JA, et al. Mechanical hypersensitivity, sympathetic sprouting, and glial activation are attenuated by local injection of corticosteroid near the lumbar ganglion in a rat model of neuropathic pain. Reg Anesth Pain Med 2011;36:56-62. [Crossref] [PubMed]
- Ammendolia C, Stuber KJ, Rok E, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev 2013.CD010712. [PubMed]
- Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976) 2005;30:936-43. [Crossref] [PubMed]
- Brown LL. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild® procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract 2012;12:333-41. [Crossref] [PubMed]
- Farooque M, Salzman MM, Ye Z. Effectiveness of Bilateral Transforaminal Epidural Steroid Injections in Degenerative Lumbar Spinal Stenosis Patients With Neurogenic Claudication: A Case Series. PM R 2017;9:26-31. [Crossref] [PubMed]


