
Perioperative dexmedetomidine and postoperative delirium in non-cardiac surgery: a meta-analysis
Introduction
Delirium is a common central nervous system disorder characterized by acute attentional deficits. It often occurs during the initial days after surgery. Many risk factors contribute to postoperative delirium. Up to this date, the pathogenesis of postoperative delirium is still unclear. One hypotheses, cholinergic theory, states that stress response theory, and inflammatory response remain to be further studied. Delirium rates among elderly surgical patients is approximately 11% to 51%, and the prevalence increases with age (1). Although the symptoms of delirium are usually transient, it negatively affects the patients’ long-term outcomes and it is associated with an increased mortality rate, cognitive dysfunction, a prolonged hospital stay, and a decrease in quality of life (1,2). As the population ages and the number of elderly patients undergoing surgery increases, preventing postoperative delirium is of utmost importance. Various pharmacologic agents (e.g., antipsychotics, acetylcholinesterase inhibitors, sleep-wake cycle regulators) have been assessed for the use in preventing postoperative delirium (3,4). However, the results from these studies are inconsistent. To date, there are no convincing pieces of evidence to support pharmacologic prevention or treatment (1).
Dexmedetomidine is a highly selective α2 adrenergic agonist that has a range of potentially beneficial effects in the postoperative period. These effects include opioid-sparing properties, decreased anesthetic requirements, and neuroprotective effects (5). For example, dexmedetomidine moderates the systemic stress response via the hypothalamic-pituitary-adrenal axis. Dexmedetomidine is a first-line drug for sedation in ICUs. Its impact on patient outcomes is still undetermined, however. In a large, randomized, controlled trial including 700 non-cardiac-surgery patients, intravenous dexmedetomidine (0.1 µg/kg/h) reduced the occurrence of postoperative delirium (6). However, another randomized, controlled trial by Deiner et al. found there to be no effect from dexmedetomidine on postoperative delirium (7). To date, there has been no meta-analysis of randomized, controlled trials which has investigated whether perioperative dexmedetomidine administration reduces postoperative delirium. Thus, we used data from ten trials to compare the effects of dexmedetomidine application versus a placebo (another sedative agent) during surgery on the risk of developing postoperative delirium.
Methods
Search strategy and selection criteria
We identified potential articles by searching the following databases up to March 20, 2019, with no language restrictions: MEDLINE, Cochrane Central Register of Controlled Trials, Embase, and PubMed. The search terms used in Embase were as follows: (“dexmedetomidine” OR “mpv1440” OR “precede” OR “dexmedetomidine hydrochloride”) AND (“delirium” OR “subacute delirium” OR “mixed origin delirium”). We also checked the references of interest and searched for trial registrations or registration agency websites to identify any other trials.
Randomized controlled trials were included if they separately assessed the effects of assigning adult patients (18 years or older) who did not undergo heart surgery, assessed postoperative delirium outcomes, compared postoperative complications of dexmedetomidine with placebo or other sedation, or followed up at least 90% of randomized patients for vital status. Trials were excluded if patients have preoperative delirium or them randomized patients to multifactorial interventions (except where factorial randomization allowed separate assessment of the effects of dexmedetomidine).
Data analysis
After removing duplicates, titles, and abstracts, the items were screened and sequentially excluded, based on the previously defined eligibility criteria. Whenever uncertainty remained after screening the title and abstract, full-text articles were scrutinized independently by two investigators, and discrepancies were resolved by consensus after discussion. Supplied data were centrally checked for completeness, plausibility, and integrity before combining them into a single database. Two independent investigators assessed the risk of bias in the included trials according to the Cochrane Collaboration’s tool for assessing the risk of bias, using primarily original trial reports. Discrepancies were resolved by consensus after discussion. Principal investigators were contacted in case of missing information.
The primary outcome was postoperative delirium. To investigate the effect of different types of sedation on delirium, we divided the control into two subgroups based on dexmedetomidine doses: patients administered a dose higher than 0.2 µg/kg/h and patients administered a dose lower than 0.2 µg/kg/h. Delirium was assessed the Confusion Assessment Method for the ICU. We used frequencies and percentages to summarize categorical variables, if relative risk is the most useful indicator of the strength of the association between exposure (intervention) and events. Therefore, we used relative risk with 95% confidence intervals (CIs) for treatment effects. All trials were analyzed separately, and respective principal investigators confirmed the results. Discrepancies were resolved by discussion.
All analyses were pre-specified unless otherwise stated. The heterogeneity of treatment effects across trials was estimated for the outcome using the Cochran’s Q test and I2 index. If heterogeneity was absent (P>0.1 and I2<50%), the fixed-effect model was used to calculate pooled effects. Otherwise, a random-effect model was used. Statistical analyses were conducted using Review Manager 5.3 (Cochran Collaboration) and Stata (Stata Corporation, College Station, TX, USA) version 12. We used Egger’s test to assess publication bias in the 10 articles included in this meta-analysis. All P values were two-sided and considered significant if less than 0.05.
Results
Description of studies
Our search retrieved 1,426 items, of which 175 were duplicates. After screening titles and abstracts, 25 reports of randomized controlled trials of interest remained and were evaluated in detail. Of these, we excluded 9 trials because they included medical or surgical ICU patients other than noncardiac surgery (8-16), three trials because other sedation was used with dexmedetomidine (17-19), one trial because patients were randomized by blocking circadian rhythms (20), one trial because patients had chronic obstructive pulmonary disorder and did not undergo surgery (21), and one trial because the control was unclear (22). As shown in Figure 1, our meta-analysis thus comprised 10 trials and 2,286 patients (23-32).
Altogether, we assigned the 2,286 patients with non-cardiac surgery to either the dexmedetomidine administration group or the control (e.g., saline placebo, other sedation) administration group. Seven studies compared dexmedetomidine with saline alone, two with propofol alone, and one with midazolam alone. Seven studies evaluated patients older than 65 years, and the other three evaluated patients older than 18 years old. Dexmedetomidine infusion rates ranged from 0.1 to 1.0 µg/kg/h. Characteristics of the various included studies are shown in Table 1. Methodological quality assessment was conducted, and the results were shown in Figure 2.
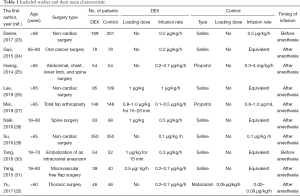
Full table
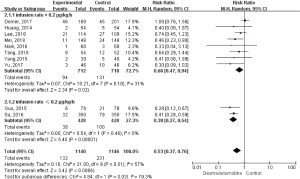
During the follow-up period, 363 postoperative delirium events were recorded. As shown in Figure 3, dexmedetomidine administration was associated with a 47% relative risk reduction of postoperative delirium (relative risk =0.53, 95% CI, 0.37 to 0.76; P=0.03). We observed significant heterogeneity in the effects among trials (I2=57%; P=0.01). The effect of dexmedetomidine administration on postoperative delirium outcomes was consistent across the prespecified participant subgroups. Further subgroup analysis was conducted based on the infusion rate of dexmetomidine, and the results proved a reduction in postoperative delirium in patients who received dexmetomidine when compared to the control. When the infusion rate was higher than 0.2 µg/kg/h had a significant 34% relative risk reduction in postoperative delirium, compared with other sedation (relative risk =0.66, 95% CI, 0.47 to 0.94; P=0.02), with no heterogeneity (I2=31%, P=0.18; Figure 3). Patients who received an infusion rate lower than 0.2 µg/kg/h had a significant relative risk reduction of 62% in postoperative delirium, compared with other sedation (relative risk =0.38, 95% CI, 0.27 to 0.54; P<0.0001), with no heterogeneity (I2=0%, P=0.46).
Publication bias and sensitivity analyses
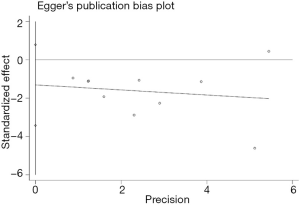
The potential publication bias was surveyed from the Egger’s plot (Figure 4) using the postoperative delirium as an endpoint. The results showed that P=0.189 without publication bias. The sensitivity analysis also showed that outcomes remained similar, regardless of which effect model was applied.
Discussion
This meta-analysis compared dexmedetomidine with a placebo control to identify the postoperative development rate of delirium in patients who underwent non-cardiac surgery. Dexmedetomidine administration was shown to have a statistically significant 47% rate of relative risk reduction of postoperative delirium.
Improvements in surgical management over the past two decades have reduced patient mortality. However, no convincing evidence yet supports pharmacologic prevention or treatment choices for postoperative delirium, which will see an increase as the elderly population increases. Dexmedetomidine is increasingly used for sedation in patients in the ICU (33). In this study, we demonstrated that dexmedetomidine sedation results in a statistically significant rate of 47% on the relative risk reduction of postoperative delirium, which is consistent with a previous study showed that compared to other sedatives, dexmedetomidine is associated with a decreased prevalence of delirium (8,34). The reason for this effect may be as follows: first of all, inflammatory response and cholinergic theory are two of the potential pathogenesis of postoperative delirium, while dexmedetomidine attenuates the inflammatory response and is lacking anticholinergic activity (35). Secondly, it is reported that compared with ICU patients without endotracheal intubation, those with endotracheal intubation have higher postoperative delirium prevalence (36). Consistently, unlike other sedations (such as propofol), dexmedetomidine could shorten extubation times (37).
Dexmedetomidine has been used by intensivists in the general practice for sedation in mechanically ventilated ICU patients at an infusion rate of between 0.2 and 1.7 µg/kg/h, with or without a loading dose (8,10,34). However, The sedative dose of dexmedetomidine used in the earlier studies (10) was associated with an increase in hypotension or bradycardia, which limits its availability in wider clinical applications (33). Previous studies demonstrated that dexmedetomidine induces dose-dependent hemodynamic changes (38). Lower doses of dexmedetomidine decreases stress response when compared to a higher dose (39). In our study, a high heterogeneity among postoperative delirium outcomes across the trials was evident. However, in a subgroup analysis of different doses of dexmedetomidine, no heterogeneity was found, which is under the total outcomes. Therefore, the potential benefits of delirium reduction need to be balanced against the increased risk of hypotension and bradycardia, particularly in patients who are at increased risk, such as those with advanced age and comorbidities. What is more, as the occurrence of postoperative delirium is at its highest during the early postoperative hours, early prophylactic pharmacological intervention is warranted (40). Deiner et al. (23) demonstrated that intraoperative dexmedetomidine does not prevent postoperative delirium, which underscores the importance of timing when administering the drug to prevent delirium. Therefore, further analysis of the timing of dexmedetomidine administration is needed.
This meta-analysis directly compares prespecified risk exposures and major outcomes, using the same definitions. However there are some limitations to our study, including the small number of trials and the limited ability to perform a meta-regression to understand the effects in different patient subgroups. Besides, we focused on postoperative delirium only and no other outcomes.
Conclusions
Perioperative dexmedetomidine administration reduced postoperative delirium outcomes in adults who underwent non-cardiac surgery. Sedation management is still an important treatment for the prevention of postoperative delirium after surgery.
Acknowledgments
Funding: This work was supported by the Guangxi Key Research and Development Program (grant no. AB18221031); the National Science Foundation of China (No. 81373498 and 81060277), the Natural Science Foundation of Guangxi (2017GXNSFBA198108), and self-Foundation of Guangxi Health Commission (Z20181017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-22. [Crossref] [PubMed]
- Abelha FJ, Luis C, Veiga D, et al. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care 2013;17:R257. [Crossref] [PubMed]
- Schrijver EJ, de Vries OJ, Verburg A, et al. Efficacy and safety of haloperidol prophylaxis for delirium prevention in older medical and surgical at-risk patients acutely admitted to hospital through the emergency department: study protocol of a multicenter, randomised, double-blind, placebo-controlled clinical trial. BMC Geriatr 2014;14:96. [Crossref] [PubMed]
- Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016;3:CD005563. [PubMed]
- Mo Y, Zimmermann AE. Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients. Ann Pharmacother 2013;47:869-76. [Crossref] [PubMed]
- Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893-902. [Crossref] [PubMed]
- Deiner S, Luo X, Lin HM, et al. Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA Surg 2017;152:e171505. [Crossref] [PubMed]
- Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644-53. [Crossref] [PubMed]
- Reade MC, O'Sullivan K, Bates S, et al. Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: A randomised open-label trial. Critical Care 2009;13:R75. [Crossref] [PubMed]
- Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically Ill patients A randomized trial. JAMA 2009;301:489-99. [Crossref] [PubMed]
- Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med 2009;35:282-90. [Crossref] [PubMed]
- Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An a priori-designed analysis of the MENDS randomized controlled trial. Crit Care 2010;14:R38. [Crossref] [PubMed]
- Wan LJ, Huang QQ, Yue JX, et al. Comparison of sedative effect of dexmedetomidine and midazolam for post-operative patients undergoing mechanical ventilation in surgical intensive care unit. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2011;23:543-6. [PubMed]
- Singh A, Ambike D, Thatte WS, et al. Dexmedetomidine versus midazolam infusion for sedation in mechanically ventilated patients in critical care setting: A randomized controlled trial. Indian Journal of Critical Care Medicine 2013;17:4.
- Reade MC, Eastwood GM, Bellomo R, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium a randomized clinical trial. JAMA 2016;315:1460-8. [Crossref] [PubMed]
- Song R, Li J, Dong C, et al. A study of using dexmedetomidine in ventilator bundle treatment in an ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:836-40. [PubMed]
- Wang K, Li C, Shi J, et al. Effects of patient-controlled intravenous analgesia with dexmedetomidine and sufentanil on postoperative cognition in elderly patients after spine surgery. Zhonghua Yi Xue Za Zhi 2015;95:2437-41. [PubMed]
- Lu X, Li J, Li T, et al. Clinical study of midazolam sequential with dexmedetomidine for agitated patients undergoing weaning to implement light sedation in intensive care unit. Chin J Traumatol 2016;19:94-6. [Crossref] [PubMed]
- Kawazoe Y, Miyamoto K, Morimoto T, et al. Effect of Dexmedetomidine on Mortality and Ventilator-Free Days in Patients Requiring Mechanical Ventilation With Sepsis: A Randomized Clinical Trial. JAMA 2017;317:1321-8. [Crossref] [PubMed]
- Li J, Dong C, Zhang H, et al. Study of prevention and control of delirium in ventilated patients by simulating blockage of circadian rhythm with sedative in intensive care unit. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016;28:50-6. [PubMed]
- Abdelgalel EF. Dexmedetomidine versus haloperidol for prevention of delirium during non-invasive mechanical ventilation. Egypt J Anaesth 2016;32:473-81. [Crossref]
- Miyamoto K, Kawazoe Y, Morimoto T, et al. Dexmedetomidine for ventilated septic patients in ICU: A multicenter randomized controlled trial. Intensive Care Med Experimental 2016.4.
- Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: A randomized clinical trial. JAMA Surg 2017;152:e171505. [Crossref] [PubMed]
- Guo Y, Sun L, Chen Z, et al. Preventive effect of dexmedetomidine on postoperative delirium in elderly patients with oral cancer. Shanghai Kou Qiang Yi Xue 2015;24:236-9. [PubMed]
- Huang F, Wang J, Yang X, et al. Sedative effects of dexmedetomidine in post-operative elder patients on mechanical ventilation. Zhonghua Yi Xue Za Zhi 2014;94:3211-5. [PubMed]
- Lee C, Lee CH, Lee G, et al. The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: A double blind randomized controlled study. J Clin Anesth 2018;47:27-32. [Crossref] [PubMed]
- Mei B, Meng G, Xu G, et al. Intraoperative Sedation With Dexmedetomidine is Superior to Propofol for Elderly Patients Undergoing Hip Arthroplasty: A Prospective Randomized Controlled Study. Clin J Pain 2018;34:811-7. [PubMed]
- Naik BI, Nemergut EC, Kazemi A, et al. The Effect of Dexmedetomidine on Postoperative Opioid Consumption and Pain after Major Spine Surgery. Anesth Analg 2016;122:1646-53. [Crossref] [PubMed]
- Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893-902. [Crossref] [PubMed]
- Tang CL, Li J, Zhang ZT, et al. Neuroprotective effect of bispectral index-guided fast-track anesthesia using sevoflurane combined with dexmedetomidine for intracranial aneurysm embolization. Neural Regen Res 2018;13:280-8. [Crossref] [PubMed]
- Yang X, Li Z, Gao C, et al. Effect of dexmedetomidine on preventing agitation and delirium after microvascular free flap surgery: A randomized, double-blind, control study. J Oral Maxillofac Surg 2015;73:1065-72. [Crossref] [PubMed]
- Yu DN, Zhu Y, Ma J, et al. Comparison of post-anesthesia delirium in elderly patients treated with dexmedetomidine and midazolam maleate after thoracic surgery. Biomedical Research (India) 2017;28:6852-5.
- Wunsch H, Kahn JM, Kramer AA, et al. Dexmedetomidine in the care of critically ill patients from 2001 to 2007: an observational cohort study. Anesthesiology 2010;113:386-94. [Crossref] [PubMed]
- Xia ZQ, Chen SQ, Yao X, et al. Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: a meta-analysis of randomized clinical trials. J Surg Res 2013;185:833-43. [Crossref] [PubMed]
- Geng J, Qian J, Cheng H, et al. The Influence of Perioperative Dexmedetomidine on Patients Undergoing Cardiac Surgery: A Meta-Analysis. PLoS One 2016;11:e0152829. [Crossref] [PubMed]
- Burkhart CS, Dell-Kuster S, Gamberini M, et al. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2010;24:555-9. [Crossref] [PubMed]
- Pasin L, Greco T, Feltracco P, et al. Dexmedetomidine as a sedative agent in critically ill patients: a meta-analysis of randomized controlled trials. PLoS One 2013;8:e82913. [Crossref] [PubMed]
- Bloor BC, Ward DS, Belleville JP, et al. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 1992;77:1134-42. [Crossref] [PubMed]
- Shamim R, Srivastava S, Rastogi A, et al. Effect of Two Different Doses of Dexmedetomidine on Stress Response in Laparoscopic Pyeloplasty: A Randomized Prospective Controlled Study. Anesth Essays Res 2017;11:1030-4. [Crossref] [PubMed]
- Shi CM, Wang DX, Chen KS, et al. Incidence and risk factors of delirium in critically ill patients after non-cardiac surgery. Chin Med J (Engl) 2010;123:993-9. [PubMed]



