Association between non-alcoholic fatty liver disease and silent carotid plaque in Chinese aged population: a cross-sectional study
Introduction
Non-alcoholic fatty liver disease (NAFLD) affects about one third of adults in both western countries and China (1,2). Although NAFLD is not a component of metabolic syndrome, existing evidence suggests a strong association between NAFLD and cardiovascular diseases (CVDs) (3). Carotid plaque is usually considered as a manifestation of subclinical atherosclerosis and can predict future cardiovascular events (4). Several studies have evaluated the association between NAFLD and subclinical atherosclerosis (5-17), but there is still controversy on this issue. Some studies (6,7,9,16), but not all (11,18), have revealed that NAFLD is an indicative index for carotid plaque, regardless of sample size, study design, degree of adjustment and ethnicity. Another cross-sectional study involving 144 subjects showed that liver fat content was significantly higher in patients without plaque than in their counterparts (P=0.009) while the liver fat content was negatively associated with plaque (OR =0.94; 95% CI, 0.89–0.99) in multivariable analysis (19). Interestingly, few studies on the association between NAFLD and carotid plaque have focused on aged adults, who have high risks for carotid plaque and stroke (20,21).
Therefore, the present study was conducted to assess the association between NAFLD and carotid plaque in about 13,000 Chinese aged adults. Because carotid plaque is related to the elevated ALT levels (22), history of CVD (23) and obesity (24), we further conducted three sensitivity analyses to test the robustness of the association between NAFLD and carotid plaque.
Methods
Study population
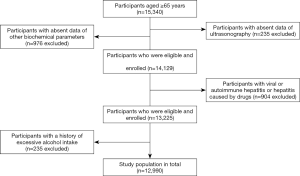
All the Chinese aged participants (≥65 years) were recruited from the Health Examination Center, Ren Ji Hospital between January 2013 and October 2018. A total of 15,340 Chinese aged adults were included. After excluding participants with incomplete information (n=1,211), viral/autoimmune hepatitis (n=613), drug-induced hepatitis (n=291) or alcohol consumption (n=235), 12,990 (7,685 men and 5,305 women) Chinese aged adults were finally included for analysis (Figure 1). The study protocol was approved by the Ethical Committee of Ren Ji Hospital (2019-112).
Assessment of NAFLD
Abdominal ultrasonography was performed using a high-resolution topographic ultrasound system with a 3.5 MHz probe (ACUSON X300, Siemens, Germany) in all the participants after overnight fast. There is evidence showing that the abdominal ultrasonographic findings closely mirror the entity of hepatic steatosis change >20% (25), and ultrasound examination is an optimal tool for the longitudinal follow-up in the elderly (26). Participants were diagnosed with NAFLD based on the presence of two of following findings: (I) diffusely increased echogenicity in the liver as compared to the kidney; (II) echogenicity attenuation; (III) poor visualization of intrahepatic structures, but absence of excessive alcohol abuse (weekly alcohol consumption ≤210 g in men and ≤140 g in women) and other liver diseases, based on the commendation of the Asia-Pacific Working Party on NAFLD and Chinese Association for the Study of Liver Disease (27,28).
Assessment of carotid plaque
Carotid plaque was also confirmed by carotid ultrasonography (Philips HDI 5000 ultrasound system equipped with a 7.5 MHz probe). Carotid ultrasonography is the primary noninvasive examination for the diagnosis and follow-up of internal carotid arteriosclerosis and carotid plaque (29). The carotid intima-media thickness (CIMT) was measured at about 1.5 cm away from the bifurcation of the common carotid artery. Carotid plaque was defined as a focal region with the thickness >1.5 mm as measured from the media adventitia interface to the lumen-intima interface or as the presence of focal wall thickening (at least 50% greater than that of the surrounding vessel wall) (30).
Assessment of other confounding factors
Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg. Body mass index (BMI) was calculated by body weight (kg) divided by the square of height (m2). Obesity was defined as BMI ≥28.0 kg/m2 based on the recommendation of Chinese Obesity Working Group (31). Blood pressure (BP) was measured using a mercury sphygmomanometer after at least 10-min rest. Information about liver function [alanine transferase (ALT), aspartate transferase (AST), alkaline phosphatase (AKP), gamma-glutamyl transferase (γ-GT), total bilirubin (TBI), direct bilirubin (DBI)], kidney function [blood urea nitrogen (BUN), creatinine (Cr), and uric acid (UC)], fasting blood glucose (FBG), lipid profiles [total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C)], and white blood cell (WBC) was also collected based on the medical records. All participants completed a health questionnaire covering issues such as history of major diseases and alcohol consumption. The excessive alcohol consumption was defined as >210 g of alcohol per week in men and >140 g per week in women (32).
Statistical analysis
Statistical analysis was performed with SAS version 9.4. Continuous variables were compared with t-test and categorical variables with Chi-square test. The association between NAFLD and carotid plaque was assessed with the multivariate logistic regression analysis. One crude model (no adjustment) and two adjusting models were constructed: in model 1, analysis was done after adjustment for age (y) and sex; in model 2, analysis was done after adjustment for BMI (kg/m2), ALT (IU/L), AST (IU/L), AKP (IU/L), γ-GT (IU/L), TBI (mmol/L), DBI (mmol/L), BUN (mmol/L), Cr (µmol/L), UC (µmol/L), TC (mmol/L), TG (mmol/L), LDL-C (mmol/L), HDL-C (mmol/L), FBG (mmol/L), and WBC (109/L). A value of two-sided P<0.05 was considered statistically significant.
We tested the interaction between NAFLD and sex, and age, with the relation to carotid plaque. To test the robustness of the results, we performed three sensitivity analyses: excluding participants with elevated serum ALT (≥75 U/L) (33), history of CVD (34), and obesity (BMI >28.0 kg/m2) (31).
Results
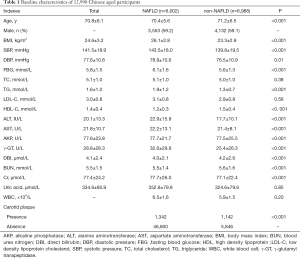
There were 6,002 aged participants with NAFLD and 6,988 aged participants without NAFLD. The mean age was 70.8±6.1 years. NAFLD participants were more likely to have higher BMI, systolic blood pressure (SBP), glucose level, TG, LDL-C, serum ALT, AST and γ-GT than those without NAFLD (Table 1).
Full table
The prevalence of carotid plaque was 19.1% in the aged population. The prevalence of carotid plaque was significantly higher in NAFLD patients than in subjects without NAFLD (22.4% vs. 16.3%, P<0.001). As compared to participants without NAFLD, NAFLD participants were more likely to develop carotid plaque (89%) (OR =1.89; 95% CI: 1.59–2.24) after adjustment for age, sex, BMI, liver and kidney function, glucose level, lipid profiles, and WBC count (Table 2, model 3).

Full table
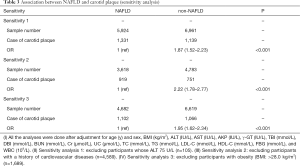
After excluding the participants with elevated ALT (≥75 U/L), a history of CVDs, and obesity (BMI >28.0 kg/m2), similar results were found (Table 3).
Full table
Discussion
In the present study, results showed NAFLD was associated with carotid plaque in 12,990 Chinese aged adults, after adjusting BMI, BP, fasting blood glucose, lipid profiles.
Similar findings have been confirmed in some studies on young adults (13,35). Participants with NAFLD had higher prevalence of subclinical atherosclerosis. In participants over 40 years, hepatic steatosis is also independently associated with the elevated CIMT after adjusting for age, gender, BMI, current smoking and regular exercise (36). Systematic reviews and meta-analysis have confirmed the strong association between hepatic steatosis and increased CIMT (9,16,37). In line with these findings in populations including adults and children (13,15-17,36,38-40), our study extended the positive correlation between NAFLD and carotid plaque to the elderly population. The absence of significant association between NAFLD and carotid plaque in other studies (11,18,19) might be related to the different diagnostic methods for NAFLD, the specific populations, the use of low frequency linear ultrasound probes, small sample size and the different ethnicity.
The most important finding in our study was that the risk of preclinical atherosclerosis persisted or even increased in asymptomatic individuals with NAFLD. Higher ALT level is also associated with increased subclinical atherosclerosis (37). In addition, elevated serum ALT level suggests more severe steatohepatitis. Thus, participants with ALT higher than 75 U/L were excluded for further analysis, and results showed the relative risk of carotid plaque detection persisted in NAFLD patients. This indicates that mild NAFLD is sensitive enough to screen out carotid plaque. Obesity is an accepted risk factor for preclinical atherosclerosis, and several studies that questioned the positive correlation between NAFLD and carotid plaque may just be a consequence of the association between obesity and atherosclerosis (11,35). Thus, obese participants were excluded for further analysis. Unexpectedly, in non-obese population, the hepatic steatosis was more closely associated with carotid plaque (OR =1.95). This unexpected higher risk was also present in NAFLD participants without prior history of CVD (OR =2.22). These results justify a necessity to screen for silent carotid plaques in the old NAFLD patients. Kang et al. (41) found that NAFLD-associated adjusted odds ratio of carotid plaque was 1.58 (95% CI: 1.31–1.86) without MetS and 1.54 (95% CI: 0.51–4.6) with MetS in outpatients without diabetes, which supported our findings. In another study, the association between NAFLD and carotid plaque was more prominent in young adults without MetS than in old adults or in those with MetS (7). These results, together with our findings, suggest that, in asymptomatic individuals, the carotid plaque may be more prevalent than in high risk populations. Thus, the screening for carotid plaque in all NAFLD individuals will be beneficial for the assessment of future atherosclerotic complications.
The large sample size and inclusion of multiple biochemical indicators were the advantages of present study. However, several limitations should be addressed. First, as a cross-sectional study, the causative association between NAFLD and carotid plaque could not be determined. Second, the drugs (such as aspirin and statin) that were reported to be associated with carotid plaque were not excluded (42) although participants with a history of CVD were excluded for further analysis. Finally, all the participants were recruited from a single center, which could not represent the general population. Thus, the results must be interpreted with caution. Further community-based prospective studies are needed to confirm our results.
In conclusion, our study indicates NAFLD is associated with carotid plaque in Chinese aged population.
Acknowledgments
Funding: This study was supported by the Construction of Key Disciplines in the Fourth Three-year Action Plan for Public Health (Health Education and Promotion) Shanghai Health and Family Planning Commission (15GWZK1001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethical Committee of Ren Ji Hospital (2019-112).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330-44. [Crossref] [PubMed]
- Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol 2017;9:715-32. [Crossref] [PubMed]
- Picardi A, Vespasiani-Gentilucci U. Association between non-alcoholic fatty liver disease and cardiovascular disease: a first message should pass. Am J Gastroenterol 2008;103:3036-8. [Crossref] [PubMed]
- Sillesen H, Sartori S, Sandholt B, et al. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging 2018;19:1042-50. [Crossref] [PubMed]
- Zhang L, Guo K, Lu J, et al. Nonalcoholic Fatty Liver Disease is Associated with Increased Carotid Intima-Media Thickness in Type 1 Diabetic Patients. Sci Rep 2016;6:26805. [Crossref] [PubMed]
- Volzke H, Robinson DM, Kleine V, et al. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol 2005;11:1848-53. [Crossref] [PubMed]
- Sinn DH, Cho SJ, Gwak GY, et al. Nonalcoholic Fatty Liver Disease for Identification of Preclinical Carotid Atherosclerosis. Medicine (Baltimore) 2016;95:e2578. [Crossref] [PubMed]
- Ramilli S, Pretolani S, Muscari A, et al. Carotid lesions in outpatients with nonalcoholic fatty liver disease. World J Gastroenterol 2009;15:4770-4. [Crossref] [PubMed]
- Madan SA, John F, Pyrsopoulos N, et al. Nonalcoholic fatty liver disease and carotid artery atherosclerosis in children and adults: a meta-analysis. Eur J Gastroenterol Hepatol 2015;27:1237-48. [Crossref] [PubMed]
- Kim HJ, Lim CW, Lee JH, et al. Gender-based differences in the relationship between fatty liver disease and atherosclerosis. Cardiovasc J Afr 2016;27:281-6. [Crossref] [PubMed]
- Dick TJ, Lesser IA, Leipsic JA, et al. The effect of obesity on the association between liver fat and carotid atherosclerosis in a multi-ethnic cohort. Atherosclerosis 2013;226:208-13. [Crossref] [PubMed]
- Gummesson A, Stromberg U, Schmidt C, et al. Non-alcoholic fatty liver disease is a strong predictor of coronary artery calcification in metabolically healthy subjects: A cross-sectional, population-based study in middle-aged subjects. PLoS One 2018;13:e0202666. [Crossref] [PubMed]
- Gill C, Vatcheva KP, Pan JJ, et al. Frequency of Nonalcoholic Fatty Liver Disease and Subclinical Atherosclerosis Among Young Mexican Americans. Am J Cardiol 2017;119:1717-22. [Crossref] [PubMed]
- Sao R, Aronow WS. Association of non-alcoholic fatty liver disease with cardiovascular disease and subclinical atherosclerosis. Arch Med Sci 2018;14:1233-44. [Crossref] [PubMed]
- Li X, Xia M, Ma H, et al. Liver fat content is associated with increased carotid atherosclerosis in a Chinese middle-aged and elderly population: the Shanghai Changfeng study. Atherosclerosis 2012;224:480-5. [Crossref] [PubMed]
- Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49:600-7. [Crossref] [PubMed]
- Kozakova M, Palombo C, Eng MP, et al. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology 2012;55:1406-15. [Crossref] [PubMed]
- Guo YC, Zhou Y, Gao X, et al. Association between Nonalcoholic Fatty Liver Disease and Carotid Artery Disease in a Community-Based Chinese Population: A Cross-Sectional Study. Chin Med J (Engl) 2018;131:2269-76. [Crossref] [PubMed]
- Loffroy R, Terriat B, Jooste V, et al. Liver fat content is negatively associated with atherosclerotic carotid plaque in type 2 diabetic patients. Quant Imaging Med Surg 2015;5:792-8. [PubMed]
- Etminan N, Chang HS, Hackenberg K, et al. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol 2019;76:588-97. [Crossref] [PubMed]
- Wang W, Jiang B, Sun H, et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017;135:759-71. [Crossref] [PubMed]
- Waluś-Miarka M, Czarnecka D, Kloch-Badelek M, et al. Carotid artery plaques - Are risk factors the same in men and women with familial hypercholesterolemia? Int J Cardiol 2017;244:290-5. [Crossref] [PubMed]
- Selwaness M, Bos D, van den Bouwhuijsen Q, et al. Carotid Atherosclerotic Plaque Characteristics on Magnetic Resonance Imaging Relate With History of Stroke and Coronary Heart Disease. Stroke 2016;47:1542-7. [Crossref] [PubMed]
- Rovella V, Anemona L, Cardellini M, et al. The role of obesity in carotid plaque instability: interaction with age, gender, and cardiovascular risk factors. Cardiovasc Diabetol 2018;17:46. [Crossref] [PubMed]
- Bril F, Ortiz-Lopez C, Lomonaco R, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 2015;35:2139-46. [Crossref] [PubMed]
- Noureddin M, Khoyilar C, Palmer SL. MRI, CT scan, and ultrasound in the diagnosis of nonalcoholic fatty liver disease. J Clin Gastroenterol 2015;49:351-2. [Crossref] [PubMed]
- Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18:163-166). J Dig Dis 2011;12:38-44. [Crossref] [PubMed]
- Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 2007;22:775-7. [Crossref] [PubMed]
- Scoutt LM, Gunabushanam G. Carotid Ultrasound. Radiol Clin North Am 2019;57:501-18. [Crossref] [PubMed]
- Mathiesen EB, Johnsen SH. Ultrasonographic measurements of subclinical carotid atherosclerosis in prediction of ischemic stroke. Acta Neurol Scand Suppl 2009.68-72. [Crossref] [PubMed]
- Zhou B. Prospective study for cut-off points of body mass index in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi 2002;23:431-4. [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. [Crossref] [PubMed]
- Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol 2017;112:18-35. [Crossref] [PubMed]
- Rasool A, Dar W, Latief M, et al. Nonalcoholic fatty liver disease as an independent risk factor for carotid atherosclerosis. Brain Circ 2017;3:35-40. [PubMed]
- VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis 2014;235:599-605. [Crossref] [PubMed]
- Zheng J, Zhou Y, Zhang K, et al. Association between nonalcoholic fatty liver disease and subclinical atherosclerosis: a cross-sectional study on population over 40 years old. BMC Cardiovasc Disord 2018;18:147. [Crossref] [PubMed]
- Kapuria D, Takyar VK, Etzion O, et al. Association of Hepatic Steatosis With Subclinical Atherosclerosis: Systematic Review and Meta-Analysis. Hepatol Commun 2018;2:873-83. [Crossref] [PubMed]
- Brea A, Mosquera D, Martin E, et al. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol 2005;25:1045-50. [Crossref] [PubMed]
- Fracanzani AL, Tiraboschi S, Pisano G, et al. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis 2016;246:208-13. [Crossref] [PubMed]
- Fracanzani AL, Petta S, Lombardi R, et al. Liver and Cardiovascular Damage in Patients With Lean Nonalcoholic Fatty Liver Disease, and Association With Visceral Obesity. Clin Gastroenterol Hepatol 2017;15:1604-11.e1. [Crossref] [PubMed]
- Kang JH, Cho KI, Kim SM, et al. Relationship between Nonalcoholic Fatty Liver Disease and Carotid Artery Atherosclerosis Beyond Metabolic Disorders in Non-Diabetic Patients. J Cardiovasc Ultrasound 2012;20:126-33. [Crossref] [PubMed]
- Goodman ZD. Phenotypes and Pathology of Drug-Induced Liver Disease. Clin Liver Dis 2017;21:89-101. [Crossref] [PubMed]