
Clinical analysis of microvascular decompression in patients with hemifacial spasm: a retrospective study
Introduction
HFS is a functional nervous disease characterized by the involuntary twitch of one side nerve and its branches. Jannetta (1) put forward the widely accepted concept of facial nerve compression from the brainstem (root exit zone, REZ). As the best treatment for HFS, MVD has become widely accepted. MVD is a functional operation, which puts forward high requirements for a neurosurgeon, not only to solve the problem of facial muscle spasm but also to reduce the occurrence of complications as much as possible, so as not to bring the new neurological deficit to the patients. The purpose of this study is to review the past patient information, analyze the characteristics of postoperative efficacy and complications, to explore better principles of MVD for the treatment of HFS.
Methods
General materials
From January 2017 to May 2018, 541 patients with HFS were recruited in the study. The primary HFS was 540 patients; secondary HFS was 1 patient. Five hundred forty patients with primary HFS had undergone MVDs. Among the 540 patients, 190 patients were male, and 350 were female; 319 patients had the left lesion and 221 patients had the right lesion. The ages ranged from 22–78 years old, and the mean age was 52.1 years old. The disease course ranged from 6 months to 12 years (lesion duration), with a mean duration of 4.5 years. According to Cohen’s classification criteria, 112 patients were in Grade 2, 305 patients were in Grade 3, and 119 patients were in Grade 4. All patients need head magnetic resonance imaging (MRI) to exclude space-occupying lesions (2). T2-weighted and 3D FIESTA MRI of the head were used to evaluate the anatomical relationship between the offending vessels and the facial nerve.
Study methods
This study excluded patients with hemifacial spasm (HFS) due to space-occupying lesion and with incomplete clinical data. All the data, such as preoperative profiles, surgical findings, postoperative images, outcomes, and complications, were recorded. All the patients were followed up through outpatient service, with an average follow-up time of two years. The study was reviewed by the experts of the hospital ethics committee and was approved as complying with the requirements and guidelines of research.
Procedure steps
All the patients underwent MVD procedure by the same neurosurgeon to avoid any bias associated with the surgeon’s experience or skills. During the operation, Nicolet endeavor CR electrophysiological monitor was used to check abnormal muscle response (AMR) to protect the cranial nerve vii and cranial nerve viii, prevent hearing loss and monitor the changes of AMR waveform. The patients were operated in the lateral position with the lesion side on the top. The incision was straight behind the ear along the hairline. The retrosigmoid approach was applied. During the craniotomy, it should be performed under the transverse sinus and behind the sigmoid sinus to prevent the injury of these important vascular tissues.
After the “+” shaped incision of the dura mater, slowly release the cerebrospinal fluid (CSF) under the microscope, cut the arachnoid tissue between the cerebellum and the pons, expose the gap between the posterior cranial nerve and the acoustic nerve, fully expose the REZ area of the root of the acoustic-facial nerve complex and its surrounding blood vessels, explore the whole course of the intracranial segment of the facial nerve, search for the offending vessels, carefully Insert Teflon pads (Shanghai Chest Medical Technological Co., Shanghai, China) in the gap between the facial nerve and the offending vessels, and recheck that there is no vascular compression on the facial nerve at the REZ area. Warm saline was applied to confirm no bleeding vessels and rinse the surgical field: suture dura mater and close incision layer by layer. The surgical pictures were shown in Figure 1.
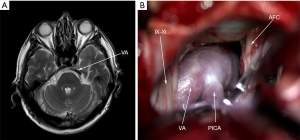
Results
Offending vessels
There were 295 patients (54.63%) with anterior inferior cerebellar artery (AICA) compression of the facial nerve, 130 patients (24.07%) with posterior inferior cerebellar artery (PICA) compression of the facial nerve, 65 patients (12.04%) with vertebral artery (VA) +PICA compression of the facial nerve, 50 patients (9.26%) with AICA+PICA compression of the facial nerve. The REZ area was the most common compression site. Moreover, 272 patients were found with facial nerve notch (FNN) at the REZ.
Surgical results
On the first day after the MVD operation, 395 patients (73.15%) were completely at once cured, 110 patients (20.37%) were partial relief, 35 patients (6.48%) were not any resolved. During the follow-up period, 455 patients (84.26%) were completely cured, 60 patients (11.11%) were partial relief, 25 patients (4.63%) were failed cure. Completely cured and partial relieved were defined as effectiveness. The effective rate was 93.52% on the first day after MVD, and 95.37% on the follow-up period.
Complications
Facial nerve palsy and hearing loss were the most common complications in the treatment of HFS after MVD. In this study, 10 patients (1.85%) were facial paralysis (FP) on the 1st day of post-operation. Twenty-five patients (4.63%) were delayed FP at the time of 1–12 w post-operation. All the patients with delayed facial palsy were completely cured in 3 months. Fourteen patients (2.59%) were hearing loss after MVD, in whom 8 patients (1.48%) had good improvement, and 6 patients (1.11%) had no changes at the period of follow-up. There was no recurrence case recorded in this study. There were 2 patients with CSF leak, and they all recovered without surgical treatment. There were 5 patients (0.93%) with postoperative fever, suggesting cranial infection, but they all recovered with proper treatment before discharge. No death occurred in all patients.
Discussion
HFS is a movement disorder characterized by intermittent, involuntary contractions of muscles innervated by the ipsilateral facial nerve. Previous studies have shown that the incidence of facial spasm is 10/100,000 per year (3,4). There are two hypotheses put forward by well-known scholars about the pathological mechanism of HFS. One was Jannetta (5,6), he believed that the neurovascular compression on the facial nerve leads to the demyelination of the facial nerve fibers, which leads to the wrong synaptic transmission in the adjacent transmission tissues. Thus, the occurrence of facial spasm resulted from the “electric short circuit”. The other was Ishikawa et al. (7); he believed that neurovascular compression increased the excitability of motor neurons of the facial nerve. The occurrence of facial spasm results from false impulses after the transshipment in the nerve nucleus. Based on the above theories, neurovascular compression on the facial nerve at the REZ was the key anatomical point. The Teflon pad was applied in the MVD to decompress the area between the offending vessels and the REZ. Therefore, Microvascular decompression (MVD) has become widely accepted as the best treatment for HFS (8,9).
Offending vessels
The common offending vessels in HFS are the AICA, PICA, VA, and their different combinations. In literature, most of the patients were single blood vessel compression on the facial nerve. In this area, the most common responsible vessel is AICA, followed by PICA (10). Recent studies (11) indicate that responsible blood vessels were not a prognostic factor of the efficacy of MVD for HFS. In this study, AICA accounted for 54.63%, and PICA accounted for 24.07% in total patients; AICA and PICA accounted for 80% of the total vessels. More attention should be paid to the compression of the facial nerve root by multiple vessels, which will increase the difficulty of the operation. One study has shown (12) that veins may be responsible for facial spasm in the case of the absence of an artery. If the possible vein was too large, the management of the decompression should be taken seriously. In our study, the symptoms of 25 patients within the follow-up period were not significantly improved. In these cases, VA, and VA+PICA as offending vessels accounted for most of the patients. The possible reasons were as follows:
- Due to atherosclerosis, the vertebral artery is difficult to move;
- Due to the small space in the posterior fossa, the space of vertebral artery displacement is limited;
- Teflon pads move with the fluctuation of CSF.
Intraoperative electrophysiological monitoring
Intraoperative neurophysiological techniques, such as AMR and lateral spread response (LSR), have been applied to check facial motor hyperexcitability during MVD. In the operation of HFS, AMR was first proposed by Møller and Jannetta in 1985 (13). AMR is an abnormal electrophysiological waveform caused by the facial nerve, which widely exists in patients with facial spasm. Many pieces of literature (14-16) had reported that the AMR wave disappeared in most patients when the neurovascular compression was relieved, and it reappeared when the neurovascular compression was restored; Therefore, intraoperative monitoring of AMR can help surgeons identify the responsible vessels, and have better postoperative outcomes. LSR monitoring is also helpful for the surgical treatment of facial spasm. Some studies have shown that LSR waveform disappears in the process of decompression, which will have a good therapeutic effect (17); when the dura mater is cut or CSF is released with the disappearance of LSR waveform, which may have a bad clinical effect (18). In our study, preoperational AMR was recorded in all patients of 540 cases. After decompressing sufficiently at the REZ area, AMR disappeared instantly in 485 cases (89.8%). AMR was still existence in 55 cases (10.2%) after MVD operations. For some patients, the AMR will not disappear at once even if the compression of blood vessels on the facial nerve is relieved during operation; these patients will be cured completely within one year after the operation. Therefore, intraoperative AMR monitoring is highly useful as an index to the effectiveness of decompression (19).
Surgical effect
Completely cured, partial relief, and failed cured were the three outcomes after MVD operation. Completely cured defined as the symptoms of facial spasm disappearance totally; partial relief defined as the symptoms of facial spasm alleviated but still occurred; failed cured defined as the symptoms of facial spasm unchanged compared with the symptoms of pre-operation. The effectiveness of MVD refers to the cases of completely cured and partial relief. The efficiency of MVD was statistically 92.9% to 98% (20,21). In some patients, the manifestation of facial spasm after the operation is not complete disappearance at once, but partial remission, gradually alleviating until complete cure in the follow-up period, which is called delayed cure. It was reported that the rate of delayed cure could be up to 36.3% (22). In this study, the rate of delayed cured was 11.11%.
Complications
FP and hearing loss are common complications after MVD operation. There are two distinct types of FP: immediate and delayed. Immediate FP is a symptom that occurs within 24 days after surgery. Some studies (23) have shown that 2.7–22.5% of the patients with immediate FP appear temporarily and can be completely relieved after the operation, and 0–8% of the patients can’t be completely relieved and become permanent FP. Delayed FP was defined as appearance after the first 24 hours after MVD, generally happens from 1 week to several weeks post-operation. The mechanism of delayed FP is still unclear. Patients with delayed FP can recover completely within a few months. The hypothesis of viral origin would be theoretically accepted that the operation may stimulate the occurrence of FP if the patient had a history of viral infection. The increase of pure tone audiogram (PTA) more than 15±5 dB after operation compared with that before the operation is considered as an abnormal change of hearing. Some scholars reported that the occurrence of the hearing deficit was 1.9–20% (23). The incidence of permanent hearing loss was 2.3%. For the elderly, the proportion of permanent hearing loss is 3.9% (24). The mechanisms of the hearing deficit are as follows: (I) damages to the acoustic nerve itself; (II) stretching of the facial-acoustic nerve complex root due to excessive retraction of the cerebellum; (III) some unascertained disturbances in microcirculation due to vasospasm.
Recurrence evaluations
It is exceedingly difficult to show the definition of the recurrence for the HFS after MVD. The cure rate is a conclusion that can only be reached after a period of follow-up. Most of the neurosurgeons for HFS thought that the conclusion of postoperative effectiveness was applicable after 1-year time of follow-up. Miller’s study analysis showed that the proportion of postoperative recurrence was 2–4% (25). In this study, the follow-up period was 1.5 to 2.5 years, on average of 2 years. Recurrence evaluation needs to be further investigated.
Limitations
As the present study is a retrospective and descriptive one in a single medical center, there were no relevant statistical analysis data provided. In the near future, multiple medical centers, statistical analysis of the follow-up results after MVD is expected.
In summary, with the improvement of microvascular skills and intraoperative electrophysiological monitoring, MVD is still the first choice for the treatment of HFS.
Acknowledgments
Funding: This work was supported by The National Natural Science Foundation of China 81573774.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was reviewed by the experts of the hospital ethics committee and was approved as complying with the requirements and guidelines of research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jannetta PJ. Trigeminal neuralgia and hemifacial spasm--etiology and definitive treatment. Trans Am Neurol Assoc 1975;100:89-91. [PubMed]
- Colosimo C, Bologna M, Lamberti S, et al. A comparative study of primary and secondary hemifacial spasm. Arch Neurol 2006;63:441-4. [Crossref] [PubMed]
- Palaram H, Carrera E, Vargas MI, et al. Familial hemifacial spasm of young-onset: Report of two cases. J Neurol Sci 2017;373:83-5. [Crossref] [PubMed]
- Lee SH, Park BJ, Shin HS, et al. Prognostic ability of intraoperative electromyographic monitoring during microvascular decompression for hemifacial spasm to predict lateral spread response outcome. J Neurosurg 2017;126:391-6. [Crossref] [PubMed]
- Jannetta PJ, Kassam A. Hemifacial spasm. J Neurol Neurosurg Psychiatry 1999;66:255-6. [Crossref] [PubMed]
- Jannetta PJ. Typical or atypical hemifacial spasm. J Neurosurg 1998;89:346-7. [PubMed]
- Ishikawa M, Tanaka Y, Watanabe E. Microvascular decompression under neuroendoscopic view in hemifacial spasm: rostral-type compression and perforator-type compression. Acta Neurochir (Wien) 2015;157:329-32; discussion 332. [Crossref] [PubMed]
- Bigder MG, Kaufmann AM. Failed microvascular decompression surgery for hemifacial spasm due to persistent neurovascular compression: an analysis of reoperations. J Neurosurg 2016;124:90-5. [Crossref] [PubMed]
- Hitchon PW, Zanaty M, Moritani T, et al. Microvascular decompression and MRI findings in trigeminal neuralgia and hemifacial spasm. A single center experience. Clin Neurol Neurosurg 2015;139:216-20. [Crossref] [PubMed]
- Qi H, Zhang W, Zhang X, et al. Microvascular Decompression Surgery for Hemifacial Spasm. J Craniofac Surg 2016;27:124-7. [Crossref] [PubMed]
- Zhao H, Tang Y, Zhang X, et al. Long-term Outcomes of Microvascular Decompression in the Treatment of Hemifacial Spasm Based on Different Offending Vessels. J Neurol Surg A Cent Eur Neurosurg 2019;80:285-90. [Crossref] [PubMed]
- Wang X, Thirumala PD, Shah A, et al. The role of vein in microvascular decompression for hemifacial spasm: a clinical analysis of 15 cases. Neurol Res 2013;35:389-94. [Crossref] [PubMed]
- Moller AR, Jannetta PJ. Microvascular decompression in hemifacial spasm: intraoperative electrophysiological observations. Neurosurgery 1985;16:612-8. [Crossref] [PubMed]
- Fukuda M, Oishi M, Takao T, et al. Monitoring of abnormal muscle response and facial motor evoked potential during microvascular decompression for hemifacial spasm. Surg Neurol Int 2012;3:118. [Crossref] [PubMed]
- Li J, Zhang Y, Zhu H, et al. Prognostic value of intra-operative abnormal muscle response monitoring during microvascular decompression for long-term outcome of hemifacial spasm. J Clin Neurosci 2012;19:44-8. [Crossref] [PubMed]
- Tobishima H, Hatayama T, Ohkuma H. Relation between the persistence of an abnormal muscle response and the long-term clinical course after microvascular decompression for hemifacial spasm. Neurol Med Chir (Tokyo) 2014;54:474-82. [Crossref] [PubMed]
- Satoh T, Onoda K, Date I. Fusion imaging of three-dimensional magnetic resonance cisternograms and angiograms for the assessment of microvascular decompression in patients with hemifacial spasms. J Neurosurg 2007;106:82-9. [Crossref] [PubMed]
- Kim CH, Kong DS, Lee JA, et al. The Potential Value of the Disappearance of the Lateral Spread Response During Microvascular Decompression for Predicting the Clinical Outcome of Hemifacial Spasms: A Prospective Study. Neurosurgery 2010;67:1581-8. [Crossref]
- Hatayama T, Kono T, Harada Y, et al. Indications and Timings of Re-operation for Residual or Recurrent Hemifacial Spasm after Microvascular Decompression: Personal Experience and Literature Review. Neurol Med Chir (Tokyo) 2015;55:663-8. [Crossref] [PubMed]
- Campero A, Herreros IC, Barrenechea I, et al. (Microvascular decompression in hemifacial spasm: 13 cases report and review of the literature). Surg Neurol Int 2016;7:S201-7. [Crossref] [PubMed]
- Chaudhry N, Srivastava A, Joshi L. Hemifacial spasm: The past, present and future. J Neurol Sci 2015;356:27-31. [Crossref] [PubMed]
- Xia L, Zhong J, Zhu J, et al. Delayed relief of hemifacial spasm after microvascular decompression. J Craniofac Surg 2015;26:408-10. [Crossref] [PubMed]
- Sindou M, Mercier P. Microvascular decompression for hemifacial spasm: Outcome on spasm and complications. A review. Neurochirurgie 2018;64:106-16. [Crossref] [PubMed]
- Youn J, Kwon S, Kim JS, et al. Safety and effectiveness of microvascular decompression for the treatment of hemifacial spasm in the elderly. Eur Neurol 2013;70:165-71. [Crossref] [PubMed]
- Miller LE, Miller VM. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: a systematic review. Br J Neurosurg 2012;26:438-44. [Crossref] [PubMed]


