
Levorphanol versus methadone use: safety considerations
Introduction
Levorphanol
In recent literature, levorphanol is referred to as “a forgotten opioid”. Levorphanol which belongs to the phenanthrene class of opioids, was first approved and marketed under the name Levo-Dromoran in 1953 (1-3). It has high affinities for all three opioid receptors (mu, delta, kappa 1, and kappa 3), where it acts as an agonist and modulates its primary nociceptive action (4,5). Levorphanol is a strong N-methyl-D-aspartate (NMDA) receptor antagonist, and also blocks the reuptake of serotonin (5-HT) and norepinephrine (NE) therefore, can be helpful to treat neuropathic pain (5). Levorphanol has a rapid onset of action achieving peak plasma concentration by 1 hour after oral administration (6). Due to its longer half-life (11–16 hours), and duration of action (6–15 hours), levorphanol can be used as a long-acting opioid (2,6).
Levorphanol undergoes phase II metabolism-glucuronidation through UDP-glucuronosyltransferase to an inactive compound levorphanol-3-glucuronide, which is renally excreted (6). Unlike methadone, cytochrome CYP450 enzyme is not required for its metabolism, and it does not bind to P-glycoprotein in the gut (2). Levorphanol like buprenorphine, has shown a ceiling effect on respiratory depression in animal models; however, its clinical relevance needs further exploration (7). Moreover, levorphanol has no known effects on QTc prolongation (8). In short, the pharmacokinetics and metabolic profile of levorphanol indicate that, it is relatively well absorbed when taken as an oral preparation, can be used as a long-acting opioid, has fewer drug interactions and risk of respiratory depression (2,7).
Levorphanol has been studied mostly in nonmalignant neuropathic pain syndromes, and limited data is available for cancer-related pain (1,9,10). However, with its unique profile, levorphanol can be considered a safe alternative to other opioids especially methadone in conditions where chronic opioid therapy is warranted (9). In addition to its role as a first-line opioid analgesic, levorphanol may be considered in opioid rotation especially in situations where opioid-induced hyperalgesia is a concern (2). Further research is needed to investigate its role in a palliative care setting and cancer pain management (10). Due to the underutilization of levorphanol, limited data exist on the safety and mortality risk as compared to well-studied opioids, including methadone (9). Summary of pharmacology and pharmacokinetics of levorphanol is outlined in Table 1.

Full table
Methadone
Methadone 6-(dimethylamino)-4,4-diphenyl-3-hepatanone hydrochloride is a synthetic mu-receptor agonist, NMDA receptor antagonist, and blocks the reuptake of 5-HT and NE (11,12). Methadone was first discovered in Germany during World War II; however, its effectiveness in the treatment of opioid dependence was not recognized until the 1950s (13). As compared to levorphanol, it has a weaker NMDA receptor antagonist effect (9). Methadone has a more complex and unpredictable metabolic profile with considerable patient variability due to gene polymorphism, P-glycoprotein dependent oral absorption, and transfer across the blood-brain barrier and gastric mucosa (11,12). In contrast to levorphanol, methadone requires the CYP450 enzyme pathway for its metabolism, which can lead to a higher risk for drug-drug and drug-food interactions (11,14). Methadone has been associated with prolongation of QTc interval in several randomized and cohort studies (15-17).
Clinically, methadone has several routes available, including rectal preparation (18). Characteristics such as high potency, low cost, and excellent oral bioavailability make it an attractive opioid in the management of chronic malignant and nonmalignant pain syndromes (18). Methadone does not have any known active metabolites; therefore, it can be given in patients with compromised renal function. Unfortunately, the use of methadone has been associated with a fivefold risk of overdose deaths as compared to other opioids, which is mainly attributed to its QTc prolongation effect (19). Methadone should be prescribed cautiously in a certain high-risk population such as females, patients with congenital cardiac channel abnormalities, and patients with low potassium and low magnesium (9,20). Summary of pharmacology and pharmacokinetics of methadone is outlined in Table 1.
Cardiovascular safety considerations
Methadone is known to cause QTc prolongation, which can predispose patients to develop cardiac arrhythmias, torsades de pointes, and sudden death (15-17,21-23). The S-isomer of methadone is a potent inhibitor of delayed-rectifier potassium current, responsible for the arrhythmogenic activity (9). There is a dose-response relationship between the degree of QTc prolongation and methadone serum concentrations (18). Current literature does not support that methadone has any direct adverse effects on the myocardium per se (18). In one study, comorbid conditions such as uncontrolled blood glucose and baseline congestive heart failure were associated with higher mortality among patients who were on methadone maintenance program (MMT) (24). The Federal Drug Administration (FDA) issued a black box warning cautioning clinicians against the fatal QTc prolongation effect of methadone (9). Cochrane review on the effectiveness of electrocardiogram (EKG) screening to prevent morbidity and mortality in patients on methadone could not draw sufficient evidence to support its use. There is a consensus in obtaining a baseline EKG in certain high-risk patients, such as those with structural heart disease, arrhythmias, family or personal history of prolonged QTc, unexplained syncope, and presence of other medications that can prolong QTc (18,25,26). It is recommended to discuss benefit versus risk of methadone therapy if QTc interval is >450 but <500 ms followed by close EKG monitoring (26). In patients with QTc >500 ms, it is recommended to either discontinue or reduce the daily methadone dose (18,26). It is recommended to obtain a follow-up EKG within 2–4 weeks of methadone initiation in patients who were previously considered high risk, those with baseline EKG >450 ms, and those with a history of syncope (18). Also, obtaining an additional EKG, when daily methadone dose reaches 30–40 and 100 mg marks, and anytime when new risk factors or clinical features of arrhythmias appear is recommended (18). It is imperative to discuss the risk and benefits of EKG monitoring when methadone is considered as a first-line agent with comfort-based goals of care or as a second-line agent with curative goals of care (18). In such scenarios, patients and their families may decide not to undergo close monitoring (18).
Levorphanol has no reported effects on QTc prolongation (9). It is considered safe in patients with preexisting risk factors as described above (9). In an observational study of patients with chronic noncancer pain treated with methadone or levorphanol the response rates were 75% and 70% respectively, and levorphanol patients did not require adjuvant analgesics and had no effect on QTc prolongation (8). A comparison of cardiac safety considerations between levorphanol and methadone is outlined in Table 2.
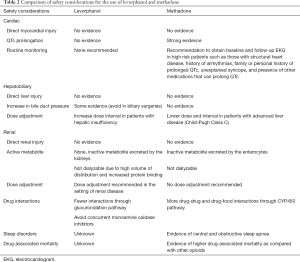
Full table
Hepatobiliary safety considerations
Methadone is not known to cause an elevation in serum liver enzymes or cause acute liver injury (27). It is highly bound to alpha-1 acid glycoprotein, which is an acute-phase protein secreted by the hepatocytes; therefore, methadone distribution and serum concentration can be affected in patients with liver disease (28). Methadone metabolism is dependent on the phase 1 enzymes (CYP450), which can be impaired in liver diseases (18). Therefore, the dose of methadone should be lowered in patients with advanced liver disease such as cirrhosis (Child-Pugh Class C) (29). Moreover, time interval should be increased during dose titration (18). Methadone use should be avoided in patients with acute fulminant hepatic failure (1,18).
Levorphanol, unlike methadone, is metabolized and excreted as levorphanol-3-glucuronide, which is an inactive metabolite (9). This metabolite is excreted renally (1). There is insufficient data on the hepatic extraction and clearances, but it is generally recommended that dose interval should be increased in patients with hepatic insufficiency (1). Levorphanol can increase pressure in the common bile duct and should be avoided in biliary surgeries (1). A comparison of hepatobiliary safety considerations between levorphanol and methadone is outlined in Table 2.
Renal safety considerations
Methadone has been considered safe in patients with renal failure (30). Methadone has inactive metabolites, which are primarily excreted in the gut and are not dialyzable in patients undergoing hemodialysis (31). Generally, no dose adjustments have been recommended in patients with renal failure (31). Metabolites of the levorphanol, on the other hand, are renally excreted and are not dialyzable due to the high volume of distribution and increased protein binding (1). It is recommended to increase dose interval in patients with compromised renal function (1). Like methadone, levorphanol can cause urinary retention due to its anticholinergic side effects (1). A comparison of renal safety considerations between levorphanol and methadone is outlined in Table 2.
Drug interactions
Methadone metabolism is dependent on cytochrome P450 enzymes. The most common enzymes are CYP2B6, CYP2C19, CYP3A4, and CYP2D6 (32,33). S-methadone, which is associated with QTc prolongation, is metabolized by CYP2B6; therefore drugs, which are inducers and inhibitors of this enzyme, can affect methadone plasma concentration, metabolism, and clearance (9). Also, CYP2B6 polymorphism can result in up to16 different allelic variants with minimal to no expression of CYP2B6, leading to high interpatient variability (32,33). Initiating a drug that acts as an inducer or discontinuing an inhibitor of CYP3A4, can decrease methadone levels (18,26). In such cases it is recommended to monitor symptoms of increased pain or opioid withdrawal (18). Patients should be instructed to use breakthrough opioids for pain or withdrawal symptoms (18). Likewise, discontinuing a drug that acts as an inducer or initiating an inhibitor of CYP3A4, will increase methadone levels, in such cases, it is recommended to empirically reduce methadone dose by 25–50%, and monitor patients for any overdose symptoms (18). A selected list of common inducers and inhibitors of CYP3A4 is outlined in Table 3.

Full table
Contrary to methadone, levorphanol has fewer drug-drug interactions since it is not dependent on CYP450 enzymes (1). Generally, drugs that inhibit glucuronidation such as tricyclic antidepressants, phenothiazines, and ranitidine can potentiate the effects of levorphanol (1). In contrast, drugs that induce glucuronidation such as carbamazepine, phenobarbital, phenytoin, and rifampin can decrease its effects (1). Concurrent use of monoamine oxidase inhibitors and levorphanol is not recommended (1). A comparison of drug interactions between levorphanol and methadone is outlined in Table 2.
Safety considerations in sleep disorders
Methadone worsens central and obstructive sleep apnea in a dose-dependent fashion (34-36). Methadone use has been associated with diminished respiratory response to PCO2, widens the alveolar-arterial oxygen gradient through hypoventilation, and directly increases apnea-hypopnea in a dose-dependent manner (34). There are no guidelines available on screening and monitoring of sleep disorders while on methadone (18). Clinicians should consider alternative options in patients with preexisting sleep apnea (18). There is no data available regarding the interactions of levorphanol and sleep-disordered breathing. A comparison of safety considerations in sleep disorders between levorphanol and methadone is outlined in Table 2.
Mortality risk
Between 1999–2010, methadone-associated deaths have disproportionately increased by 600% as compared to an increase in deaths due to other opioids of 138% (19). When used as a first-line opioid in the treatment of chronic pain among hospitalized patients, the out of hospital mortality increased to 46% during the follow-up period (37). In the majority of the published data on methadone-associated death, it is difficult to differentiate the cause of death from respiratory depression and fatal arrhythmias (38). Nevertheless; methadone safety concerns are widely accepted due to its unique pharmacology (18,26,39). Recently, various organizations have published consensus guidelines to promote safer use of methadone (18,26,39). In recent years, methadone-associated deaths have declined, which might be associated with increased awareness and safe prescription patterns (40).
The mortality risk with levorphanol is unknown because it has not been widely used (9). A comparison of mortality risk between levorphanol and methadone is outlined in Table 2.
Drug availability and cost consideration
In the United States, methadone is manufactured by Ascent Pharmaceuticals INC (41). It is available in 5 and 10 mg oral preparations (42). Methadone is widely available and is covered by Medicare and most of the insurance plans (42). The average retail price of 5 mg oral tablet (#90) is $31.28 (42).
Levorphanol is manufactured by Sentynl Therapeutics INC, and Virtus Pharmaceuticals (41). It is available in 1, 2 and 3 mg oral preparations (43). In our experience, levorphanol is not readily available in the majority of the pharmacies and may have regional differences (10). The average retail price of 2 mg oral tablet (#90) is $4,490.42 (43).
Conclusions
Levorphanol has a more predictable pharmacokinetic profile, with a shorter half-life and prolonged duration of action as compared to methadone. Unlike methadone, it undergoes glucuronidation to an inactive metabolite, which is renally excreted. Levorphanol has no QTc prolongation risk, fewer drug-drug interactions, and like buprenorphine may have a ceiling effect on respiratory depression. Despite the safer profile of levorphanol, the limited knowledge regarding its use in the palliative care setting, lack of easy availability along with the high cost of the drug may make prescribing levorphanol a challenge as compared to methadone. Further research is needed to investigate the role of levorphanol in the setting of palliative care and cancer pain which may subsequently make the drug more accessible and affordable for our patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mellar P. Davis) for the series “Opioid Utility the Other Half of Equianalgesia” published in Annals of Palliative Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Opioid Utility the Other Half of Equianalgesia” was commissioned by the editorial office without any funding or sponsorship. A Reddy: Study drug (levorphanol) was provided free of cost by Sentynl Therapeutics INC for Dr. Reddy’s separate prospective open-label study, which is currently in progress. A Haider has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Prommer E. Levorphanol: the forgotten opioid. Support Care Cancer 2007;15:259-64. [Crossref] [PubMed]
- Gudin J, Fudin J, Nalamachu S. Levorphanol use: past, present and future. Postgrad Med 2016;128:46-53. [Crossref] [PubMed]
- McNulty JP. Can levorphanol be used like methadone for intractable refractory pain? J Palliat Med 2007;10:293-6. [Crossref] [PubMed]
- Stringer M, Makin MK, Miles J, et al. d-Morphine, but not l-morphine, has low micromolar affinity for the non-competitive N-methyl-d-aspartate site in rat forebrain. Possible clinical implications for the management of neuropathic pain. Neurosci Lett 2000;295:21-4. [Crossref] [PubMed]
- Codd EE, Shank RP, Schupsky JJ, et al. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther 1995;274:1263-70. [PubMed]
- Dixon R, Crews T, Inturrisi C, et al. Levorphanol: pharmacokinetics and steady-state plasma concentrations in patients with pain. Res Commun Chem Pathol Pharmacol 1983;41:3-17. [PubMed]
- Le Rouzic V, Narayan A, Hunkle A, et al. Pharmacological Characterization of Levorphanol, a G-Protein Biased Opioid Analgesic. Anesth Analg 2019;128:365-73. [Crossref] [PubMed]
- McNulty JP. Chronic pain: levorphanol, methadone, and the N-methyl-D-aspartate receptor. J Palliat Med 2009;12:765-6. [Crossref] [PubMed]
- Pham TC, Fudin J, Raffa RB. Is levorphanol a better option than methadone? Pain Med 2015;16:1673-9. [Crossref] [PubMed]
- Reddy A, Ng A, Mallipeddi T, et al. Levorphanol for Treatment of Intractable Neuropathic Pain in Cancer Patients. J Palliat Med 2018;21:399-402. [Crossref] [PubMed]
- Ferrari A, Coccia CP, Bertolini A, et al. Methadone--metabolism, pharmacokinetics and interactions. Pharmacol Res 2004;50:551-9. [Crossref] [PubMed]
- Lugo RA, Satterfield KL, Kern SE. Pharmacokinetics of methadone. J Pain Palliat Care Pharmacother 2005;19:13-24. [Crossref] [PubMed]
- Payte JT. A brief history of methadone in the treatment of opioid dependence: a personal perspective. J Psychoactive Drugs 1991;23:103-7. [Crossref] [PubMed]
- Fudin J, Fontenelle DV, Fudin HR, et al. Potential P-glycoprotein pharmacokinetic interaction of telaprevir with morphine or methadone. J Pain Palliat Care Pharmacother 2013;27:261-7. [Crossref] [PubMed]
- Krantz MJ. Heterogeneous impact of methadone on the QTc interval: what are the practical implications? J Addict Dis 2008;27:5-9. [Crossref] [PubMed]
- Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med 2007;167:2469-75. [Crossref] [PubMed]
- Ehret GB, Voide C, Gex-Fabry M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: high frequency in hospitalized patients and risk factors. Arch Intern Med 2006;166:1280-7. [Crossref] [PubMed]
- McPherson ML, Walker KA, Davis MP, et al. Safe and Appropriate Use of Methadone in Hospice and Palliative Care: Expert Consensus White Paper. J Pain Symptom Manage. 2019;57:635-45.e4. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep 2012;61:493-7. [PubMed]
- Raffa RB, Burmeister JJ, Yuvasheva E, et al. QTc interval prolongation by d-propoxyphene: what about other analgesics? Expert Opin Pharmacother 2012;13:1397-409. [Crossref] [PubMed]
- Milroy CM, Forrest AR. Methadone deaths: a toxicological analysis. J Clin Pathol 2000;53:277-81. [Crossref] [PubMed]
- Clark JC, Milroy CM, Forrest AR. Deaths from methadone use. J Clin Forensic Med 1995;2:143-4. [Crossref] [PubMed]
- Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf 2005;14:747-53. [Crossref] [PubMed]
- Fareed A, Vayalapalli S, Scheinberg K, et al. QTc interval prolongation for patients in methadone maintenance treatment: a five years follow-up study. Am J Drug Alcohol Abuse 2013;39:235-40. [Crossref] [PubMed]
- Pani PP, Trogu E, Maremmani I, et al. QTc interval screening for cardiac risk in methadone treatment of opioid dependence. Cochrane Database Syst Rev 2013.CD008939. [PubMed]
- Chou R, Cruciani RA, Fiellin DA, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain 2014;15:321-37. [Crossref] [PubMed]
- Methadone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
- Viani A, Rizzo G, Carrai M, et al. Interindividual variability in the concentrations of albumin and alpha-1-acid glycoprotein in patients with renal or liver disease, newborns and healthy subjects: implications for binding of drugs. Int J Clin Pharmacol Ther Toxicol 1992;30:128-33. [PubMed]
- Davis M. Cholestasis and endogenous opioids: liver disease and exogenous opioid pharmacokinetics. Clin Pharmacokinet 2007;46:825-50. [Crossref] [PubMed]
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage 2004;28:497-504. [Crossref] [PubMed]
- Kreek MJ, Schecter AJ, Gutjahr CL, et al. Methadone use in patients with chronic renal disease. Drug Alcohol Depend 1980;5:197-205. [Crossref] [PubMed]
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138:103-41. [Crossref] [PubMed]
- Kharasch ED, Regina KJ, Blood J, et al. Methadone Pharmacogenetics: CYP2B6 Polymorphisms Determine Plasma Concentrations, Clearance, and Metabolism. Anesthesiology 2015;123:1142-53. [Crossref] [PubMed]
- Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest 2005;128:1348-56. [Crossref] [PubMed]
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev 2007;11:35-46. [Crossref] [PubMed]
- Webster LR, Choi Y, Desai H, et al. Sleep-disordered breathing and chronic opioid therapy. Pain Med 2008;9:425-32. [Crossref] [PubMed]
- Ray WA, Chung CP, Murray KT, et al. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med 2015;175:420-7. [Crossref] [PubMed]
- Chou R, Weimer MB, Dana T. Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline. J Pain 2014;15:338-65. [Crossref] [PubMed]
- Chou R. 2009 Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn 2009;119:469-77. [PubMed]
- Faul M, Bohm M, Alexander C. Methadone Prescribing and Overdose and the Association with Medicaid Preferred Drug List Policies - United States, 2007-2014. MMWR Morb Mortal Wkly Rep 2017;66:320-3. [Crossref] [PubMed]
- . Available online: https://www.fda.gov/media/124011/downloadAdministration USFD.
- GoodRx.com. Methadone 2019. Available online: https://www.goodrx.com/methadone?label_override=methadone&form=tablet&dosage=5mg&quantity=90
- GoodRx.com. Levorphanol 2019. Available online: https://www.goodrx.com/levorphanol


