CT image guided thermal ablation techniques for palliation of painful bone metastases
Introduction
Painful bone metastases are a common cause of morbidity in patients with metastatic cancer, especially when combined with possible neural compression and pathologic fractures. Several solid cancers are associated with bone involvement. Up to seventy percent of cancer patients develop bone metastases. Treatment of local disease may reduce the pain of these patients who, in most cases, have a life expectancy of months. Such treatment must be fast, safe, effective and tolerable.
A number of treatment methods are available that have variable success and complications. Radiation therapy is the preferred treatment in this setting, but other modalities such as chemotherapy, hormonal therapy, radiopharmaceutical therapy and surgery, alone or in combination with non-steroid anti-inflammatory drugs (NSAIDs), opioids and adjuvant drugs, are used for pain palliation.
Radiofrequency ablation (RFA) is a relatively new method for the treatment of painful bone metastases. RFA has been employed for the treatment of hepatocellular carcinoma (HCC), liver metastases, renal and lung tumours, as well as for the treatment of osteoid osteoma, for which it has become the treatment of choice (1-3). Competing methods include chemical ablation (with ethanol or acetic acid) and thermal therapies, such as with laser, microwave, ultrasound and cryoablation (4).
Microwave ablation (MWA) uses electromagnetic waves in the microwave energy spectrum (300 MHz to 300 GHz) to produce tissue-heating effects. The oscillation of polar molecules produces frictional heating, ultimately generating tissue necrosis within solid tumors. It is generally used for the treatment and/or palliation of solid tumors in patients who are nonsurgical candidate.
MWA has emerged as a newer ablation modality and an addition to the arsenal of minimally invasive cancer care. The purported benefits of MWA over RFA and laser include a larger and faster volume of tissue heating with a given application. Unlike RFA, MWA does not rely on an electrical circuit allowing for multiple applicators to be used simultaneously.
The aim of this study was to demonstrate and compare the effectiveness of computed tomography (CT) guided RFA and MWA of painful bone metastases.
Materials and methods
In our study a total of 45 patients with bone metastases were concluded. There were 29 men and 16 women. Their ages ranged between 36 and 90 years (mean ± standard deviation: 65.43±10.56 years). Thirty patients were treated with RFA and fifteen with MWA, all under CT guidance. All treated lesions were osteolytic with a combination of bone destruction and a soft tissue mass. Bone metastases were diagnosed by bone scintigraphy and spiral CT. The diagnosis was confirmed with a core biopsy obtained at the beginning of the procedure. Their topographical distribution and the originating primary malignancies according to the ablation method are presented in Table 1. In our study the most common treated metastases originated from lung cancer.
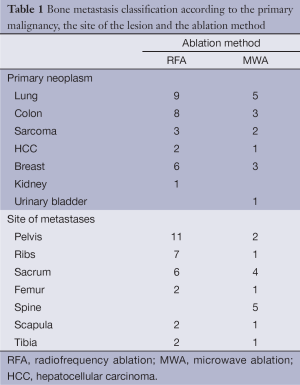
Full table
Lesion diameter was between 2 and 9 cm (mean ± SD: 3.9±2.6 cm). For sizes over 3 cm, up to three electrode placements were performed. Previously obtained imaging examinations were evaluated for lesion and feasibility of electrode positioning and ablation. Lesions located in proximity to the spinal cord and major nerves (less than 1 cm) were excluded from ablation treatment. All patients selected to undergo an ablation had Brief Pain Inventory (BPI) score above 4, weren’t appropriate candidates for irradiation or surgery, didn’t respond or had major complications to chemotherapy and/or radiation therapy, had life expectancy greater than two months and those patients who preferred this treatment over other alternatives. Before the procedure all patients were informed and consent was obtained in each case.
Pain was assessed with the BPI on a numerical rating scale where 0 indicates no pain, and 10 indicates worst pain imaginable. The use of analgesics was recorded the day before the procedure.
Pre-procedural blood tests included measurements of hemoglobin concentration, international normalized ratio, partial thromboplastin time and platelet count. Exclusion criteria included only coagulopathy, INR >1.5 or a platelet count of less than 60,000/mm3. The procedure was performed under conscious sedation (administration of 3 mg of bromazepam PO and 50 mg of pethidine hydrochloric acid intramurally, 45 min prior to the procedure). All patients were placed in the appropriate position (prone, supine, or lateral, depending on the site of the lesion) and a scan of the desired area with a 5 mm slice thickness was performed. The imaging modality of choice for the percutaneous electrode guidance was spiral CT (Somatom Emotion Duo System, Siemens, Munchen, Germany). All procedures were performed by the same interventional radiologist (LT) with a 20-year experience in CT-guided percutaneous interventions.
The lesion’s exact location and depth, in relation to the overlying skin, was determined on CT. The skin was then prepared with povidone iodine (10%) solution and local anaesthesia (15 mL of 2% lidocaine hydrochloride solution) was administered.
RFA was performed with a RITA Model 1500X® electrosurgical generator, 250 W power, 460 kHz frequency (RITA Medical Systems, Mountain View, CA, USA) and a seven-array, 2- to 3-cm multitined electrode for lesions smaller than 3 cm (10 out of 30), or a nine-array multitined electrode for larger lesions (20 out of 30). MWA was performed with AMICA-GEM microwave generator, output up to 100 W continuous wave at 2,450 MHz (AMICA-GEM, NH Hospital Service, Rome, Italy) connected to a 14- or 16-gauge coaxial antenna endowed with a miniaturized sleeve choke to reduce back heating effects and increase the sphericity of the ablated area. The electrode tip was inserted to approximately 1 cm from the centre of the target. The electrodes were then deployed slowly, taking into account the need to ablate the lesion—bone interface. RF ablation time was 7-12 min at an energy level of 90-110 W, with the target goal temperature set to 80-110 °C, while ablation time with MW was 4-8 min. All number of electrode placements, total ablation times, total energy delivered to the target and lesion temperatures achieved were recorded. Technically successful ablation is considered to be when the lesion is treated according to protocol and completely covered (5).
After each session a dual-phase spiral CT examination with intravenous contrast medium was performed in order to evaluate immediate response, as confirmed by lack of contrast enhancement and low lesion attenuation values.
Patients were hospitalised and observed for 24-hour monitoring and were discharged the day after, provided that no complication occurred. Analgesics were administered if required. Before patient discharge the pain was re-evaluated with the BPI score. Post-ablation assessment with BPI score and report of the use of analgesics was performed with telephone interview one, four and eight weeks after the ablation.
Results
Before ablation therapy all 45 patients received NSAIDs, opioids or an opioid/NSAID combination. One week after treatment only 6 out of 45 patients were treated with NSAIDs, or a combination of NSAID/low-dose opioids. After four and eight weeks only 3 out of 45 patients received painkillers. One patient died during the 8-week follow-up due to his primary malignancy.
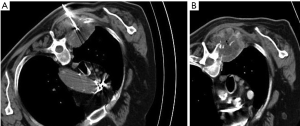
Lesions smaller than 5 cm (36 out of 45 cases) were treated with one placement of the ablation electrode while lesions larger than 5 cm (9 out of 45 cases) required up to three placements (Figure 1). The total procedure time ranged between 23 and 47 min (mean ± SD: 37±11 min) (Table 2). Post-treatment CT revealed a good immediate response to all patients. None major (hemorrhage, thrombosis of proximal veins) or minor (skin burns, post ablation syndrome) complication occurred.

Full table
Thirty nine out of forty five patients (86.6%) reported early pain reduction. Six patients reported no pain reduction during the first 24 h after the procedure and were treated with analgesics (opioids or an opioid/NSAID combination). None of the patients reported pain increase. Prior to the procedure, the mean past day BPI score for worst pain was 8.2, mean pain was 6.7, and mean pain interference with daily life 7.4. After RF ablation these scores were reduced to 7.3, 4.5 and 6.3 one day after the session, dropped to 4.7, 3.1 and 3.9 after one week, to 3.4, 1.9 and 2 after four weeks and to 2, 1.4 and 1.7 after eight weeks respectively. After MW ablation therapy these scores dropped to 7.4, 4.4 and 6 one day after the procedure to 4.6, 3 and 3.9 after one week, to 3.4, 2, and 1.8 after four weeks and 1.9, 1.5 and 1.6 after eight weeks.
These results revealed equal BPI score decrease for both RF and MW ablation, with subsequent improvement in the life quality of all patients from the first week after the procedure up to the 8-week follow-up (Figure 2). The mean past day BPI score for worst pain, for average pain and for pain interference in daily life improved in comparison to preprocedural symptoms (P<0.001, paired t-test).
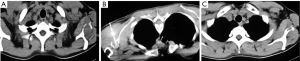
Great difference was noted in the duration of the ablation session between the two methods. Mean ablation time for RFA was 9.5 minutes while for MWA was 4.5 minutes achieving the same clinical result. Microwaves heat biological tissues rapidly in comparison with RFA.
Discussion
Bone metastases are difficult in handling. A number of treatment options are available, including NSAIDs, opioids, adjuvant drugs medications, radiation therapy, chemotherapy, hormonal therapy, radiopharmaceutical therapy, imaging guided percutaneous ablation, surgery and vertebroplasty.
Medication is the first line of treatment. NSAIDs and adjuvant drugs represent basic medication, potentially followed by NSAID/low-dose opioid combinations, and finally increasing the opioid dose.
Radiation therapy is another treatment option that may also be employed in pathologic or impending fractures (6). Approximately 70% of patients undergoing radiation therapy will experience pain relief after between 2-3 days and up to four weeks after treatment. However, radiation therapy may also cause complications, mostly from damage of adjacent soft tissues (6).
Chemotherapy and radiopharmaceutical therapy are systemic methods of metastatic cells treatment with low sensitivity, not well tolerated and associated with many complications. Radiopharmaceutical therapy can be more useful in treating patients with multifocal bone metastases. It has been reported that radiopharmaceuticals proved efficient in pain palliation mostly in bone metastases from breast, prostate and perhaps small cell lung cancer. Radiopharmaceutical agents vary with regard to the analgesic efficacy, duration of pain palliation, ability to repeat treatments, toxicity and expense (7).
Ablation refers to the local destruction of the tumor by application of either chemical agents (ethanol, acetic acid), or local deposition of some form of energy (radiofrequency, laser, microwave, ultrasound and cryoablation). Image-guided RF ablation is currently used for the treatment of various tumours with good results. According to preliminary results of Callstrom et al. (8) after treating 12 patients concluded that this modality provides an effective and safe alternative method of pain palliation in patients with osteolytic metastases. A multicentre study involving 43 patients with painful osseous metastases was carried out by Goetz et al. (9) showed again significant reduction of pain and decrease in the use of opioids, with only minor complications. Confirmation of the method’s efficiency and safety was reported with the study published by Thanos et al. in 2008 (10).
The low conductivity and poor thermal conduction in bone are limiting factors for RFA. Nevertheless, many sites perform RF ablation to treat osteoid osteomas and palliation of painful bone metastases (11). Due to bone’s low conductivity and relative permittivity, microwaves may penetrate deeper, be less affected by tissue heating or dessication and be more effective for heating bone tumors than RF energy. However few reports of MWA for bone tumors have been published in the scientific literature and much more study is needed to determine whether these predictions are true (12). According to our study, although with a limited number of patients, patients treated with microwaves presented similar clinical response with those treated with RFA (13). So the theoretically advantage of microwaves wasn’t confirmed.
Unlike RFA, MWA does not rely on an electrical circuit allowing for multiple applicators to be used simultaneously. The fact that no pads and currents are dispersed through the patient’s body makes MWA more safe and simple procedure.
The proposed mechanisms ablation decreases pain may involve: pain transmission inhibition by destroying sensory nerve fibres in the periosteum and bone cortex; reduction of lesion volume with decreased stimulation of sensory nerve fibres; destruction of tumour cells that are producing nerve-stimulating cytokines [tumour necrosis factor-alpha (TNF-α), interleukins, etc.] and inhibition of osteoclast activity (14,15). In our patients, we observed a considerable reduction of pain, improvement of life quality (as measured by the BPI score) and decrease in the use of analgesic medications possibly greater than others reported in previous studies (10).
Patients treated with microwaves experienced a faster ablation procedure compared to those treated with RFA. Microwave heat biological tissues very rapidly and allow complete coagulative treatments of bigger lesions in a shorter time. The reduction of RFA procedural time is limited by both the time needed to achieve the optimal target temperature and the size of the lesion, because more than one deployment is required in larger bone metastases. Although a few patients reported mild discomfort during the ablation, none of the sessions was forced to stop due to patient distress (16).
None possible complication, including infection, haemorrhage, neurological complications, skin burns, or post-ablation syndrome (low-grade fevers ≤37.8 °C, myalgias, and malaise for up to one week after the procedure) was reported. Pain reduction was fast and occurred within the first 24 h for some and during the first week in the majority of the patients. This appears to be a fairly well-tolerated procedure and the combination of conscious sedation and local anaesthesia is adequate for its needs.
The use of RFA in spinal metastases is limited due to the proximity with the spinal cord and nerve root. In most series lesions within 1 cm of the spinal cord, involving the posterior wall, with cortical bone destruction were considered as contraindication of RFA (17,18). All spinal metastases in our series were located in the anterior vertebral body and were treated with MWA. The clinical result was excellent with total necrosis of the lesion, decrease of the BPI score immediately after the ablation until the 8-week follow-up. The ablation duration was minimized to a mean time of four minutes. None complication occurred in our patients.
The follow-up period for this study was eight weeks, a period sufficient to demonstrate that ablation provides effective palliation. There is, however, a need for randomised prospective studies, to evaluate the methods and to compare them with other treatment modalities.
Conclusions
While RF ablation has been an effective tool for treating tumors of the liver, lung, kidney and bone, has certain limitations. RF heating is limited in areas of high perfusion (kidney and liver), in tissues with poor electrical and thermal conductivity (lung and bone), and in areas near large heat sinks (liver, lung and kidney). Microwaves offer all of the same benefits as RF thermal ablation, but do not depend on tissue properties and have the ability to heat faster in a larger volume. The fact remains that microwave systems for clinical use are still very expensive in comparison with RFA systems. This has hampered the study of microwaves for tumor ablation and resulted in many speculations about its efficacy without a great deal of scientific data to stand on. Commercial and academic development is ongoing to create MWA systems easier to use with lower cost. Until then, RF energy will most likely remain the dominant modality for thermal tumor ablation in the liver, lung, kidney and bone.
Acknowledgements
All human and animal studies have been approved by the hospital’s ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Disclosure: The authors declare no conflict of interest.
References
- Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol 2001;12:1135-48. [PubMed]
- Rosenthal DI, Hornicek FJ, Torriani M, et al. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology 2003;229:171-5. [PubMed]
- Gangi A, Alizadeh H, Wong L, et al. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology 2007;242:293-301. [PubMed]
- Callstrom MR, Atwell TD, Charboneau JW, et al. Painful metastases involving bone: percutaneous image-guided cryoablation--prospective trial interim analysis. Radiology 2006;241:572-80. [PubMed]
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005;16:765-78. [PubMed]
- Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res 2003;(415 Suppl):S158-64.. [PubMed]
- Bauman G, Charette M, Reid R, et al. Radiopharmaceuticals for the palliation of painful bone metastasis-a systemic review. Radiother Oncol 2005;75:258-70. [PubMed]
- Callstrom MR, Charboneau JW. Percutaneous ablation: safe, effective treatment of bone tumors. Oncology (Williston Park) 2005;19:22-6. [PubMed]
- Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol 2004;22:300-6. [PubMed]
- Thanos L, Mylona S, Galani P, et al. Radiofrequency ablation of osseous metastases for the palliation of pain. Skeletal Radiol 2008;37:189-94. [PubMed]
- Kojima H, Tanigawa N, Kariya S, et al. Clinical assessment of percutaneous radiofrequency ablation for painful metastatic bone tumors. Cardiovasc Intervent Radiol 2006;29:1022-6. [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25 Suppl 1:S69-83. [PubMed]
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007;10:120-31. [PubMed]
- Mannion RJ, Woolf CJ. Pain mechanisms and management: a central perspective. Clin J Pain 2000;16:S144-56. [PubMed]
- Honore P, Luger NM, Sabino MA, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 2000;6:521-8. [PubMed]
- Callstrom MR, Charboneau JW, Goetz MP, et al. Image-guided ablation of painful metastatic bone tumors: a new and effective approach to a difficult problem. Skeletal Radiol 2006;35:1-15. [PubMed]
- Nakatsuka A, Yamakado K, Takaki H, et al. Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: a prospective study. Cardiovasc Intervent Radiol 2009;32:70-5. [PubMed]
- Buy X, Basile A, Bierry G, et al. Saline-infused bipolar radiofrequency ablation of high-risk spinal and paraspinal neoplasms. AJR Am J Roentgenol 2006;186:S322-6. [PubMed]


