Multiple organ dysfunction caused by severe fungal infection: report of one case and a literature review
Case presentation
The patient, a 40-year-old male butcher, presented in the Department of Respiratory Medicine of our center on January 18, 2014 due to “fever for more than 10 days”. Chest CT and abdominal color Doppler ultrasound at admission showed no abnormality. Routine blood test showed a white blood cell (WBC) count of 10.96×109/L and a hemoglobin concentration of 102 g/L. Biochemical testing showed the following: potassium, 3.27 mmol/L; total bilirubin, 57.73 µmol/L; direct bilirubin, 19.11 µmol/L; indirect bilirubin, 38.62 µmol/L. Testing for calcitonin, respiratory pathogens, anti-Ro antibodies, rheumatoid factors, typhoid, and autoimmune antibodies showed no obvious abnormalities. He received symptomatic treatments including anti-infective therapy with moxifloxacin hydrochloride, fluid replacement, and physical cooling. However, the fever chills persisted, and his body temperature fluctuated between 39 and 40.2 °C. The patient’s body temperature did not decrease after intermittent intravenous infusion of hydrocortisone and methylprednisolone. Imipenem and cilastatin sodium for injection + azithromycin were then used to treat bacterial infections.
Biopsy
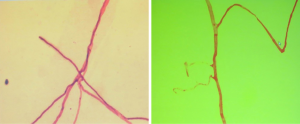
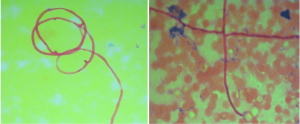
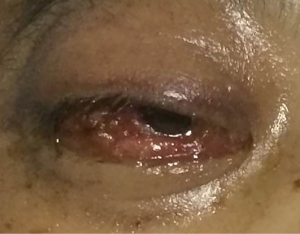
Blood smear indicated a decrease in the granulocyte-erythrocyte ratio and a proportion of atypical lymphocytes of 1.5%. The results of bone marrow biopsy + culture and repeated blood bacterial culture were not obtained. Since the patient was a butcher, his blood sample was also sent to the local center for disease control (CDC) to test for anti-Brucella antibodies, which showed negative results. The patient went unconscious on the afternoon of January 21, and had paroxysmal convulsions on the right-side of his body, vertical gaze palsies, and incontinence of urine and feces, which lasted about 1–2 minutes. Plain and enhanced head MRI and FLAIR showed no obvious abnormalities, although there was a slight abnormality on electroencephalography (EEG). A second routine blood test showed the following: WBC count, 20.37×109/L; hemoglobin concentration, 88 g/L; neutrophil-to-lymphocyte ratio (NLR), 93.40%. A biochemical test showed the following: sodium, 128.30 mmol/L; total bilirubin, 31.01 µmol/L; direct bilirubin, 11.34 µmol/L; indirect bilirubin, 19.67 µmol/L; alanine aminotransferase, 84.60 IU/L; aspartate aminotransferase, 51.10 IU/L; D-dimer, 0.88 mg/L. A lumbar puncture showed a cerebrospinal fluid pressure of 180 mmHg and a nucleated cell count of 19×106/L; also, Pandy’s test showed a negative result. A biochemical test of the cerebrospinal fluid showed the following: chlorine, 109.75 mmol/L; glucose, 5.23 mmol/L; total protein, 0.27 g/L. India ink method and smear testing showed negative results. The level of calcitonin was normal. On January 23, 2014, the possibility of intracranial infection was considered after a hospital-wide consultation. On the morning of January 24, the patient developed respiratory failure, which was managed by tracheal intubation and ventilator-assisted breathing. After being transferred to our department on the morning of January 24, a hospital-wide consultation was organized again. Meanwhile, the blood culture results and bone marrow smear were obtained, which confirmed the presence of fungi (as shown in Figures 1,2). In addition, the liver enzymes were significantly elevated. Since the patient was critically ill, he received anti-fungal treatment with amphotericin B and caspofungin acetate (Cancidas). He was also treated with human immunoglobulin (Ph4) intravenous injection to enhance his immune status, thymalfasin injection (Zadaxin) to control his epilepsy symptoms, piperacillin sodium and tazobactam sodium injection to fight against infections, reduced glutathione and polyene phosphatidylcholine injection to protect the liver, and ganciclovir injection to remove viruses. In addition to the above, multidisciplinary treatments including appropriate dehydration, nutritional support, and use of brain cell protection agent were offered. On January 28, a tracheotomy was performed. After January 31, the patient did not experience obvious epileptic seizures, and these stopped completely by February 2. Because the patient’s body temperature normalized, no obvious dry or wet rales were heard on the lung auscultation, and the indicators of inflammation were normal. Antibiotics were stopped on February 9, and mechanical ventilation was stopped on February 10. On February 16, the patient again developed high fever, and the indicators for inflammation rose once more. Ceftriaxone sodium or injection was used as an anti-infective treatment, but the fever persisted after 3 days. Piperacillin sodium and tazobactam sodium injection was then used instead. On February 23, the clinical laboratory called to inform our department that Gram-negative bacilli were detected after culture of the blood sample was submitted on February 22. Meropenem for injection was then used to prevent sepsis. To treat anemia, 1.5 U (300 mL) and 2 U (400 mL) of the type A erythrocyte suspension were transfused on February 17 and 23, respectively. However, on the night of February 25, the patient developed red eyes, exposed conjunctiva, and purulent secretions in both eyes (Figure 3). These symptoms were more prominent in the right eye, and gradually the right eye became swollen. On the morning of the February 26, the right eye was obviously swollen, the right lower eyelid was prolapsed outside the eye, the eyelids were red, the left eye was slightly swollen, the conjunctiva was red, and the patient had a fever. After consultations with specialists, the possibility of inflammation was considered, and the possibility of bleeding could not be ruled out. Computed tomography (CT) of the eyelids revealed that the right eyelid and the eye ring were obviously swollen, and flaky high-density shadows were visible in the right posterior walls of the bilateral eyeballs, which could have been a result of bleeding or other factors. The findings of eye color ultrasonography included the following: (I) thickened eyelids, with uneven echoes; (II) retinal detachment in both eyes; (III) vitreous and omental turbidity; (IV) possible choroidal detachment on the right posterior wall of the right eye; (V) a subretinal horizontal spot in left eye, which could have been inflammatory deposits or hemorrhage. The patient’s body temperature remained high (above 39 °C, with the peak temperature at 39.8 °C). CT of the eyelids on February 28, 2014, revealed that the right eyelid and the eye ring were obviously swollen, and flaky high-density shadows were visible in the right posterior walls of the bilateral eyeballs, which could have been a result of bleeding or other factors. The findings of eye color ultrasonography showed the following: (I) thickened eyelids, with uneven echoes; (II) retinal detachment in both eyes; (III) vitreous and omental turbidity; (IV) possible choroidal detachment on the right posterior wall of the right eye; (V) a subretinal horizontal spot in the left eye, which could have been inflammatory deposits or hemorrhage. Anti-infective therapy with meropenem for injection for 5 days and with sulbactam/self-prepared minocycline for 3 days failed. Then, a 50 mg injection of tigecycline every 12 hours and a 3 g injection of cefoperazone sodium and sulbactam sodium every 8 hours were intravenously administered as anti-infective therapies and accompanied by eye drainage. Three days later, the swelling of the right eye was alleviated; 10 days later, the body temperature returned to normal. The tracheal tube was closed on March 5, 2014. On March 15, 2014, the tracheal catheter was withdrawn after thorough evaluation. Neurological examination showed the patient was conscious and could basically answer questions; the conjunctiva in the right eye was red but without exudate; the right pupil was about 7.0 mm in diameter, and its light reflex disappeared; the left pupil was about 3 mm in diameter, and its light reflex was sluggish; the remaining cranial nerves showed no obvious abnormality. On March 26, 2014, plain head MRI + FLAIR showed multiple blurred spotty or patchy abnormal signals on the bilateral frontal/temporal lobes, insular lobe, basal ganglia, lateral ventricle, and semi-oval center, which might have been due to encephalitis or other reasons; compared with the previous MRI + FLAIR findings, the scope of the lesions was increased. Also, bilateral sinus and mastoid inflammation was observed. At present, the general condition of the patient is acceptable. He has no particular discomfort like dizziness or headaches, and he can walk around with the help of his family. Neurological examinations showed that he was conscious; the eyes had no sensitivity to light; the right pupil was 0.3 cm in diameter and irregular in shape, and its light reflex had disappeared; the left pupil was 0.4 cm in diameter, and its light reflex had also disappeared. The tongue was centered, and he could move his limbs. Babinski sign appeared transiently on the right side. The neck was soft, and no Kernig’s sign was detected. Chest CT revealed a small number of patchy shadows at the basal segment of the right lower lung, with most of them being old lesions; bilateral pleural thickening and bilateral pleural effusions were also found, which were milder than in the previous film. EEG showed moderate abnormalities. The initial pressure of cerebrospinal fluid was 160 mmH2O. Biochemical tests revealed the glucose level was increased (4.92 mmol/L). Routine tests showed no obvious abnormalities. The total dose of amphotericin B reached 2,025 mg by April 15th. The patient could walk with the support of his family, and his left eye had sensitivity to light. Neurological examinations showed that he was conscious; the eyes had no sensitivity to light; the right pupil was 0.3 cm in diameter and irregular in shape with no functional light reflex; the left pupil was 0.4 cm in diameter, and its light reflex was also not functional. The tongue was centered, and he could move his limbs. Tendon reflex was positive. Chaddock sign was detected on the right side. The neck was soft, and Kernig’s sign was suspected. The patient was discharged on April 15. A follow-up visit arranged 3 months later showed that the patient had completely normal body temperature and had returned to relatively normal day-to-day life; however, the vision of his two eyes had not recovered.
Discussion
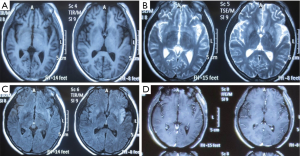
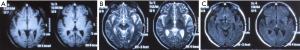
The patient presented with fever at admission, which was accompanied mainly by respiratory symptoms, followed by a loss of consciousness and seizures (mainly status epilepticus). While the above symptoms were effectively controlled after treatments, gastrointestinal symptoms, optic nerve damage, and symptoms of blood disorders (mainly anemia) occurred. Based on the patient’s occupation and disease etiologies, we sent the blood samples to the local CDC to rule out the possibility of HEN1 infection and other infectious diseases. After the admission, the disease condition progressed rapidly. Bone marrow smear and blood smear were soon tested, which identified a filamentous fungus (as shown in Figures 1,2), although the specific type of the fungus was unknown.
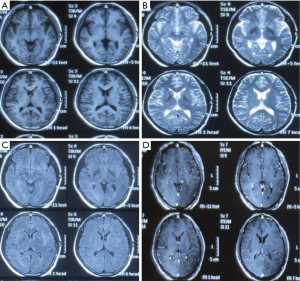
Identification of the infection of filamentous fungi is difficult. In our current case, after 3 samples failed to identify the specific type of fungus, testing in 5 batches of bone marrow smears and blood smears suggested a clinically rare pathogen was present. The patient’s main manifestations were fever and nervous system damage, which quickly spread to various systems of the whole body, indicating that the mass reproduction of the pathogen caused serious infections, resulting in rapid progression of the disease and thus damage to multiple organs. History-taking revealed that the patient’s left middle finger had been stabbed by a pig bone spur on January 5th and was not properly treated; the left middle finger soon became red and swollen in the afternoon. The patient suffered from redness and pain of the parts below the left wrist after the morning of January 6. On January 8, the whole left arm became red, swollen, and painful, and the patient experienced fever (about 38 °C); no special treatment was offered. On January 10, the redness and pain in the left upper limb was relieved, but the fever persisted, accompanied by fatigue, poor appetite, and other discomforts. He thought the condition was a common cold and did not seek medical treatment. On January 16, the patient visited a local hospital due to persistent fever and fatigue. Routine blood testing showed that the WBC count increased, whereas no special abnormalities were observed on chest X-ray; he was then treated with oral antibiotics. As the injury occurred before the development of his condition, we speculated that the disease was blood-borne after the finger trauma. It was unfortunate that the patient and his family did not keep the pig bone spur and collect a local sample at the injury site. The rapid spread of this severe disease in multiple systems and the favorable working environment for the growth of the pathogen indicated that the pathogenicity of the pathogen was related to the environment. According to the literature (1), fungi are found in soils, water, and spoiled plants, with septum and horny branched hyphae; however, the mycelium of the mucor is banded, and there is no (or only a small number of) septum. Fungi usually enter the human body through the respiratory tract and are then disseminated to the central nervous system (CNS) via the blood. However, fungi (including Exophiala dermatitidis and E. rostratum) can directly infect the CNS or bone marrow of even immunocompetent individuals in cases of surgery, trauma, intravenous drug use, or exposure to contaminated medical supplies (2). Fungi can also be spread from adjacent tissues (sinus structures, mastoids, or eyelids) to the CNS. Because the fever in the patient was not specific, it could have easily led to misdiagnosis. Notably, the patient first experienced fever, followed by neurological symptoms. Lumbar puncture suggested normal cerebrospinal fluid pressure, while routine cerebrospinal fluid test, biochemical tests, and smear testing did not reveal any abnormality, with the initial abnormal findings on head imaging not being obvious (Figures 4-6).Since there was no etiological evidence, symptomatic anti-tuberculosis treatment was considered. However, blood culture, blood smear, and bone marrow smear reported the presence of a filamentous fungus. We also had considered that the disease might be caused by fungal contamination; however, this possibility was ruled out since the pathogen was identified in all 3 batches of blood samples, and it was impossible for the specimens to appear in bone marrow smear and bone marrow culture. Therefore, the diagnosis of fungal infection could be confirmed. Also, it suggests that special attention should be paid to infections of an unknown origin. In addition, 3 sessions of head MRI + FLAIR after his admission revealed that the lesion was mainly located at the border between the gray matter and white matter. Although no abscess was formed, the possibility of blood-borne dissemination could not be ruled out (3,4).
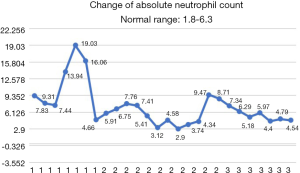
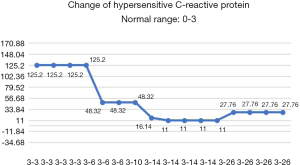
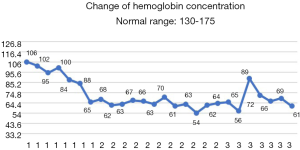
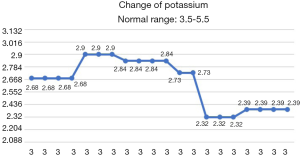
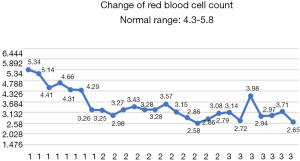
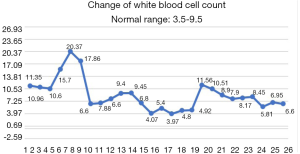
After the patient was transferred to our department, the treatment was not based on specialist treatment, and the patient’s condition changed rapidly, as shown by the following test results in Figures 7-13.
After potent comprehensive treatments, the patient’s epileptic symptoms did not recur, the liver function was corrected, and the infection was also controlled. After successfully being withdrawn from mechanical ventilator support, the patient recovered consciousness. All the above-mentioned indicators improved. Unfortunately, the light reflex of the bilateral pupils and the visibility of the choroidal vessels on the ocular fundus decreased, with the patient showing poor response to hand motions. We speculated that the optic nerve had also been affected, as confirmed by the subsequent local symptoms of the patient’s eyes. Fungi can spread from adjacent tissues (sinus structures, mastoids, or eyelids) to the CNS. Infection of the ethmoid sinus can cause cavernous sinus thrombosis through the emissary veins. The hyphae in the ethmoid sinus can enter the periorbital region via the lamina papyracea, threatening the eyeballs, extraocular muscles, and posterior eye structures (including the optic nerve). According to the literature, the mucormycosis of ethmoid sinus affects all structures in its invasion pathway, including the eyelids, eyeballs, bones, and brain tissues (4,5). The affected tissues may be abraded, scratched, or biopsied to collect specimens. It has been reported that the disseminated Fusarium infection can cause bilateral endophthalmitis and thus lead to blindness. The incidence of chorioretinitis caused by blood-borne disseminated Fusarium infection is also higher than that caused by other fungal infections (6,7). Because disseminated Fusarium infection often causes mycobacteria and skin nodules, rapid diagnosis and treatment can prevent their progression into encephalitis and endophthalmitis (8). In our current case, due to the high body temperature and severe inflammatory reaction, no eye discharge specimen was collected for bacteriological tests as a biopsy might have led to the further increase of local inflammatory reaction. Although the local symptoms were rapidly controlled after potent anti-infective treatment, we regret not conducting this test.
Fungal meningitis is not uncommon in clinical settings, and its clinical treatment is difficult (9); however, the successful treatment of multiple systemic impairment caused by blood-borne fungal infection was a rare and challenging experience for us due to the lack of relevant guidelines. On the basis of early detection, use of antifungal drugs, multidisciplinary evaluation, and management of immune damage, the combined anti-fungal treatment (amphotericin B for killing the fungus and caspofungin for inhibiting the fungus) achieved remarkable effectiveness; furthermore, the use of multidisciplinary therapies soon controlled the disease condition. According to our experience, amphotericin B begins at a small dose, and then the dose is escalated progressively to a maintenance dose of 25–30 mg per day, with a total dose of 2000 mg. In our current case, 24-hour uninterrupted maintenance was applied. Caspofungin was administered for 4 weeks. The infection in his eyes was successfully controlled by local drainage and symptomatic anti-infective treatments. His liver function recovered to normal after his epilepsy symptoms did not recur following a series of multidisciplinary treatments including liver protection and anti-epilepsy therapy. The endotracheal tube was withdrawn smoothly. The cognitive function was good, and there were no residual neurological sequelae. At last, the patient could walk by himself. He has been followed up for 6 consecutive months, during which time no symptoms have recurred. Despite these successful treatments, it is regrettable that the patient’s vision was seriously impaired by endophthalmitis. To improve our clinical practice, detailed history-taking, prompt analysis of examination/test findings, and early diagnosis and treatment are essential. We will continue to collect and summarize any similar cases in our work.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- McCarthy M, Rosengart A, Schuetz AN, et al. Mold infections of the central nervous system. N Engl J Med 2014;371:150-60. [Crossref] [PubMed]
- Perfect JR. Iatrogenic fungal meningitis: tragedy repeated. Ann Intern Med 2012;157:825-6. [Crossref] [PubMed]
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis 2012;54 Suppl1:S16-22. [Crossref] [PubMed]
- Hopkins RJ, Rothman M, Fiore A, et al. Cerebral mucormycosis associated with intravenous drug use: three case reports and review. Clin Infect Dis 1994;19:1133-7. [Crossref] [PubMed]
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54 Suppl 1:S23-34. [Crossref] [PubMed]
- Martino P, Gastaldi R, Raccah R, et al. Clinical patterns of Fusarium infections in immunocompromised patients. J Infect 1994;28 Suppl 1:7-15. [Crossref] [PubMed]
- Steinberg GK, Britt RH, Enzmann DR, et al. Fusarium brain abscess: case report. J Neurosurg 1983;58:598-601. [Crossref] [PubMed]
- Lamaris GA, Esmaeli B, Chamilos G, et al. Fungal endophthalmitis in a tertiary care cancer center: a review of 23 cases. Eur J Clin Microbiol Infect Dis 2008;27:343-7. [Crossref] [PubMed]
- Smith RM, Schaefer MK, Kainer MA, et al. Fungal infections associated with contaminated methylprednisolone injections. N Engl J Med 2013;369:1598-609. [Crossref] [PubMed]