
A retrospective review for the use of palliative sedation in a regional hospital in Hong Kong
Introduction
Palliative sedation is defined as monitored use of medication intended to induce a state of decreased or absent awareness (unconsciousness) to relieve the burden of otherwise intractable suffering in a manner that is ethically acceptable to the patient, family and health-care providers (1). Internationally, there are multiple published guidelines from different organizations recommending on how to deliver sedation in palliative care settings under different cultural and ethical backgrounds (1-5). Though different guidelines may have different recommendation regarding the timing of initiation, level of sedation and concurrent life-sustaining treatment, one common theme among these guidelines, is to ensure palliative sedation is given for the right indication adequately and proportionally, which means that the consciousness is lowered only to the level necessary and sufficient to achieve the desired level of symptom alleviation to relief patient’s suffering but not to hasten death (2,6,7).
In Hong Kong, there is no consensus across the territory on how to administer palliative sedation in patients with imminent death. Moreover, all the published guidelines and medication recommendations are developed mostly in Caucasian settings, which may not be taken into account the cultural aspect in Chinese. Previously our unit reported that the use of end of life care pathway (ECP) was feasible in Chinese population (8), and relatives were satisfied with the symptom control achieved during the last days of life (9). Since there are no published data on the dosage of drugs used in palliative sedation in Chinese population, we would like to report the practice in our unit, and to compare our results with those in international guidelines, and to study the factors associated with successful sedation in Chinese population.
Methods
Characteristic of palliative care unit, Tuen Mun Hospital
Tuen Mun Hospital is a public regional hospital in Hong Kong which serves about 1 million population, with 45 in-patient palliative care beds managed by oncologists. Patients who deteriorated during their hospital stay are assessed by multi-disciplinary team and recruited into ECP if they have irreversible deterioration due to cancer or its complication, and if 2 out of 4 of the following criteria applied:
- bed bound;
- semi-comatose;
- only able to take sips of fluid;
- no longer able to take tablets.
According to the symptoms, oncologist-in-charge could initiate palliative sedation if clinically indicated. In case if patients do not fulfill the above criteria, ECP can be omitted as per oncologist’s discretion.
Study design
All patients with histological or radiological evidence of malignancy who died in palliative care ward from 1st July 2017 to 30th September 2017 were screened. All patients, who received continuous midazolam infusion were included in our retrospective review. Patients’ demographic data, cancer disease status, laboratory results, interview records and availability of advance directives were retrieved from electronic patient records and in-patient hospital notes. Pre-existing symptoms and treatment received before palliative sedation were all reviewed. All drug records including the dose of midazolam and other concomitant drugs and duration of palliative sedation were assessed. Sedation level and symptoms during the end of life period were obtained from standardized ECP charts. Survival data estimated from the day of admission to our department until death was recorded.
Successful sedation in our study was defined as patients had adequate symptom control in more than or equal to 80% of the sedated period. Patients’ symptoms were assessed at least 3 times a day by nursing staff and once daily by the oncologist in charge. Symptoms were recorded using standardized form in ECP in 65 out of 81 patients. In case if patients were not recruited into ECP, symptom improvement was retrospectively reviewed from in-patient records. The time with adequate symptom control was divided by the total sedated time to obtain the percentage time with adequate symptom control.
Statistical analysis
Statistical analysis was performed using SPSS® version 22.0. Frequency distributions were used to describe the demographic data and the distribution of each variable. A Chi-squared test was used to make comparisons for categorical variables, and Fisher’s exact test was used if sample size criteria were not met for Chi-squared approximation. The Kaplan-Meier curve and log-rank test were used to compare the survival time between sedated and non-sedated patients. A P value of less than 0.05 was considered statistically significant.
The relationship between successful sedation and associated factors including baseline characteristics, laboratory results, reasons for sedation and concurrent symptoms, dosage of midazolam and other concomitant drugs were analyzed using univariate analysis. Statistically significant factors were then analyzed by multivariate analysis to determine whether there was correlation with the outcome of successful sedation.
Results
Demographic data
One-hundred and eighty patients died in our palliative care ward from 1st July 2017 to 30th September 2017. Eighty-one patients received continuous infusion of midazolam during their hospital stay, resulting in a prevalence of 45% of patients received palliative sedation during the study period. One patient improved and sedation was stopped, but later deteriorated again with sedation re-initiated. A total of 82 episodes with 339 patient-days were studied. The median age was 68 years old (range, 37–93) for the sedated group and 65 years old (range, 36–91) for the non-sedated group. Median survival upon admission to the oncology bed was 11 days in the sedated group versus 9 days in the non-sedated group. The most common diagnoses were lung cancer, colorectal cancer as well as hepatobiliary and pancreatic cancers in both groups. There was no statistical difference in the baseline characteristics of both groups, except that was a small tendency to have more lung cancer in the sedated group, which was statistically insignificant (Table 1). There was also no significant difference in survival time after admission to oncology wards in both groups as shown in the Kaplan-Meier curve in Figure 1.
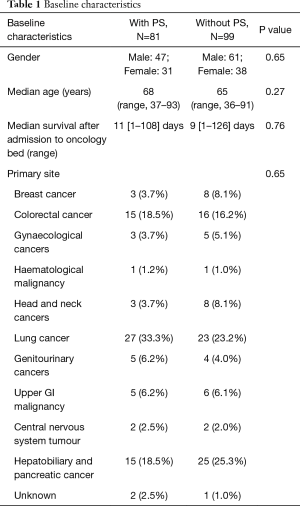
Full table
The baseline characteristics of the group of sedated patients were listed in Table 2. In the sedated group, the most common metastatic sites were lung or pleural metastases (46.9%) followed by liver metastases (30.9%) and bone metastases (30.9%). Median Karnofsky performance scale (KPS) was 40 upon admission to palliative care ward, with KPS ranged from 20–70. Baseline organ function before sedation was assessed. Sixty-six patients had blood taken for liver function test within 2 weeks before initiation of palliative sedation. Twenty-six out of 66 patients (39.3%) of them had serum total bilirubin level greater than the upper limit of normal. Fifty-two patients had blood taken within 2 weeks before the initiation of palliative sedation. Twenty-two out of 52 (42.3%) patients had serum creatinine level greater than the upper limit of normal.
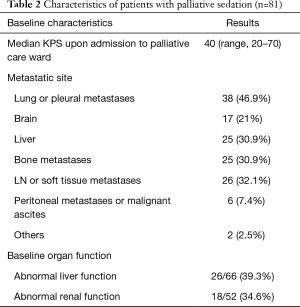
Full table
Details of sedation
Duration of palliative sedation
Details of sedation including duration of sedation, reasons for sedation and symptoms indicated for sedation were listed in Table 3. The median time for patients on sedation was 32.33 hours (range, 2.91–1,240 hours). Thirty-two patients (39%) passed away within 24 hours since initiation of palliative sedation, while 56 patients (68.4%) passed away within 3 days since initiation of palliative sedation. There were 10 patients (7.5%) who received palliative sedation for more than 7 days, and the maximum time of sedation was 51.8 days in our patient series. Two patients (2.5%) stopped sedation and resumed afterwards. In one patient, she received light sedation for controlling of dyspnea due to severe chest infection. Her condition improved with antibiotics; therefore, sedation was stopped. However, she deteriorated again due to disease progression and palliative sedation was resumed 3 weeks later till death. In another patient, relatives requested to terminate the sedation when the patient deteriorated, hoping her general condition might improve. Yet, the patient’s general condition remained poor, and symptoms worsen after termination of sedation, thus relatives agreed to resume sedation for comfort.
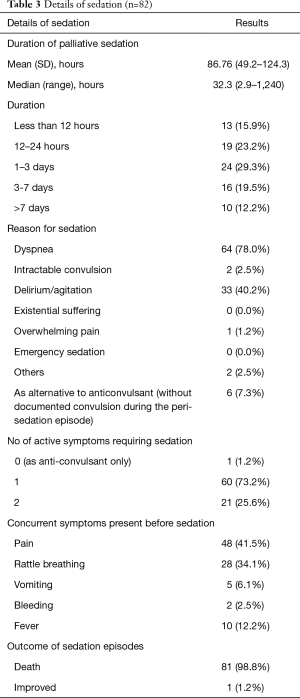
Full table
Reason for sedation
Dyspnea was the most common cause for palliative sedation in 64 out of 82 (78.0%) sedation episodes, followed by delirium or agitation in 33 out of 82 (40.9%) sedation episodes. In 26 (31.7%) episodes, patients had 2 major symptoms required sedation. Five patients (6.1%) required midazolam for palliative sedation for other baseline symptoms and anticonvulsants. One patient (1.2%) received midazolam for its anti-epileptic property alone. The most common concurrent symptom presented before sedation was pain in 34 out of 82 (41.5%) episodes and death rattle in 28 out of 82 episodes (34.1%).
Types of palliative sedation, supportive measures after sedation, and depth of sedation
All 82 episodes in our cohort received proportional and continuous sedation, which means the sedating drug was titrated up gradually to the desired depth of sedation. In 333 patients-days (98.2%), palliative sedation was given via subcutaneous route while remaining 5 patient-days (1.8%), palliative sedation was given via intravenous route. It was contributed by one patient who received continuous intravenous infusion of midazolam and morphine which was prescribed in acute medical ward by the physician in charge before transferal to oncology unit. It was then changed to subcutaneous route for patient's comfort upon transferal to palliative care ward. Patients would continue oral feeding until intolerance. If they already received tube feeding before their condition deteriorated, feeding would be continued even if patients were sedated for symptoms control and would only be terminated if they are unfit for enteral feeding. In our locality, patients’ relatives expected their loved ones to have some form of artificial hydration even in the end of life. Therefore, it is our usual practice to continue hydration using subcutaneous infusion for patient’s comfort. Other medical treatments would be continued if deemed necessary.
The sedation score currently used in our unit is modified from Pasero Opioid Sedation Scale (POSS). Since most of our patients received palliative sedation when approaching the end of life, monitoring of sedation score would be terminated by that time. The conscious level would then be assessed using descriptive terms as charted in our ECP template. Sedation levels are therefore grouped as conscious, confused or agitated, semiconscious or unconscious in the study as shown in Table 4. Most patients were kept either semi-conscious or unconscious after sedation in 79 patient-days (23.3%) and 118 patient days (34.8%) respectively. Some patients remained conscious, which were defined as alert or slightly drowsy, in 99 patient-days (29.2%). However, some patients remained restless or confused after sedation in 43 patient-days (12.7%).
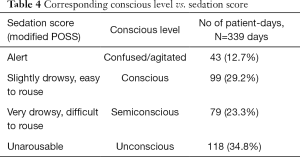
Full table
Drug of choice and concomitant drug use
All patients received midazolam for sedation with a mean dose of 10 milligram per day (range, 5–45 mg). The estimated dose per kg of body weight was determined by the last available body weight during out-patient visits within 3 months before the patient succumbed. In 6 of our patients, body weight was not available as they were either transferred to oncology ward directly from other units, or too frail to have their body weight measured in recent few months before admission. Mean dose of midazolam per kilogram per day was 0.16 mg/kg/day (range, 0.04–0.64 mg/kg/day). The ratio of doses of intravenous midazolam to subcutaneous midazolam was taken as 1:1; according to published literature on pharmacokinetics (10). The most common concomitant drug class used was opioid in 87.6% of assessed patient-days, with morphine in 244 patient-days (71.9%) and fentanyl in 53 patient-days (15.3%) respectively. Hyoscine butyl bromide was the second commonly co-administered therapeutic agent, which was presented in 258 patient-days (76.1%), followed by haloperidol in 162 patient-days (47.8%). Midazolam was concurrently administered with other drugs in 335 patient-days (98.2%), most commonly with two additional therapeutic agents as in 168 patient-days (49.6%), and three additional therapeutic agents in 108 patient-days (31.9%). The details of medication used for sedation and dosage were listed in Table 5.
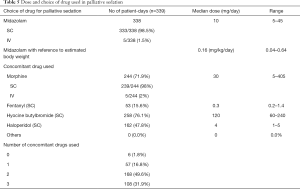
Full table
Procedure on sedation and discussion with patients or relatives
In all sedation episodes, reasonable options for treatment of reversible causes and use of non-sedating therapy for vigorous control of symptoms had been attempted before the initiation of palliative sedation. Only 1 out of 82 sedation episode showed improvement and successfully stopped the treatment.
In our patient cohort, only 7 out of 81 patients (8.6%) had advance directive for refusal of cardiopulmonary resuscitation signed before admission. All of these seven patients deteriorated during the admission and were not able to discuss the option of sedation before their deterioration. In the remaining 74 patients, 17 of them (21.0%) could discuss treatment options and agreed to proceed with palliative sedation for comfort. Discussion with relatives was done in 79 out of 81 patients (97.5%). One patient had no relatives available for discussion, while the reason remains unknown in the other patient. After sedation, all except one patient were able to have a quiet time to say goodbye with their loved ones. All patient relatives received patient updates and psychosocial care from nursing colleagues.
Rate of successful sedation and factors associated with successful sedation
Out of 81 patients, 3 patients shortly succumbed within the same nursing shift. These patients were excluded from calculating the percentage time of having adequate symptom control during the sedated period. The median percentage time with adequate symptom control was 83.5% (range, 0–100%) in our cohort. In patients who achieved adequate symptom control, the median duration to achieve adequate sedation after initiation of palliative sedation was 5.9 hours (range, 0–130 hours). In 43 out of 78 patients (55.1%), their symptoms were adequately controlled more than 80% of the sedated time. Remaining 35 patients (44.9%) had the patients’ symptom control time less than 80% of the sedated time. Multiple factors were assessed to look for the possible factors associated with successful symptom control.
In univariate analysis, multiple factors including the absence of shortness of breath, higher bilirubin level and lower dose of midazolam required were associated with higher probability of adequate symptom control. The baseline characteristics including age, gender, primary diagnosis and site of distant metastases did not statistically significantly affect the sedation outcome. The doses of concomitant drugs used were also not significantly associated with successful symptom control. These factors were listed in Table 6.
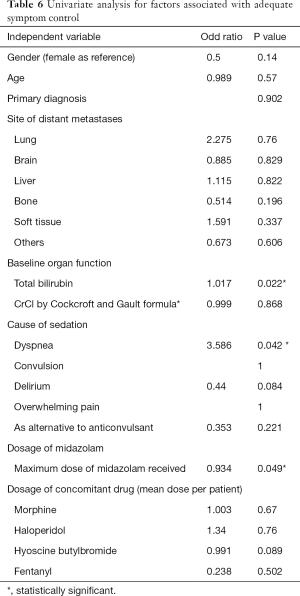
Full table
These significant factors were then analyzed using logistic regression. Deranged liver function, surrogated by the level of serum total bilirubin, was the only statistically significant factor that affected the outcome of sedation after multivariate analysis. Patients who had higher total bilirubin level would have a higher chance of having adequate symptom control during the sedated period with odds ratio of 1.02 (95% CI, 1.002–1.032, P=0.02). The maximum dose of midazolam received by individual patients, or the absence of shortness of breath became statistically insignificant after multivariate analysis.
Discussion
Terminal cancer patients experience a variety of symptoms in the last hours to days of life, including delirium, agitation, anxiety, terminal restlessness, dyspnea, pain, vomiting, and psychological and physical distress. Occasionally, these symptoms may become refractory and unable to be controlled by supportive and palliative therapies specifically targeted to these symptoms alone. Palliative sedation therapy is one of the potential solutions for this situation. However, the practice varies among different localities and different cultural backgrounds.
This is the first retrospective review on reporting the practice of palliative sedation in Hong Kong. The prevalence of sedation in our case-cohort is about 45%, which is similar to the prevalence report in the literature ranges from 10% to 50%, with a median estimate of 20–30% (11-15). Our reported prevalence was higher than the reported prevalence in a Taiwanese study in 2001 (27.9%), in a Singaporean study (22.6% at 48 hours before death) in 2012, and a Chinese study in 2015 (33.6%) (13-15). Though Asian population may share a similar cultural background, the substantial difference in the prevalence rate and clinical practice could be due to different understanding of palliative sedation. In a German study, Stiel et al. attempted to review the factors associated with high versus low sedation rate estimates in Palliative and Hospice Care in Germany, and supported that even with the same clinical scenarios there would be variation in defining palliative sedation. They also concluded that there were no associations between health care professionals’ demographic data and profession-related background and their reported palliative sedation rate in their practice (16).
Some physicians suggested palliative sedation would have the ‘Doctrine of Double Effect’, which means that if doing something morally good has a morally bad side-effect, it is ethically acceptable to do it provided the bad side-effect was not intended. Though randomized control trials are not feasible in assessing the effect of palliative sedation, in recently published literature in different settings, sedation was not associated with negative impact on survival. This result was also reproduced in our patient cohort, illustrated by the similar survival since admission to palliative care ward in Figure 1. This re-emphasized that palliative sedation can be safely used for terminal symptom management without hastening death during the end of life.
Therapeutic choice for palliative sedation
Midazolam is a short-acting benzodiazepine, with a rapid onset time of about 50 min in subcutaneous route (8). It has anxiolytic, anti-epileptic and muscle relaxant properties, and can be administered either subcutaneously or intravenously in bolus form or continuous infusion. It is reported as the most commonly used drug because of its favorable properties, including high potential for sedation, low risk of respiratory depression at sedative doses, a wide safety margin and its short half-life which offers better titrating responsiveness. Multiple dosage suggestions have been recommended in different guidelines, ranging from initial dose with 0.5 to 1 milligram per hour, and salvage as needed dose ranging from 1 to 5 milligram, followed by continuous sedation at 1 to 20 milligram/hour. British palliative care formulary guideline recommends 5 to 10 milligram stat dose for initiation of palliative sedation, followed by continuous infusion with doses range from 30 to 120 milligram per day (11). In our case series, most of our patients received midazolam infusion at a smaller dose than the recommended dose in guidelines, with a median dose of 10 milligram over 24 hours, which was slightly higher than the dose reported by the Singaporean study with median 5 milligram over 24 hours (15). It could be related to a few reasons. Body build of our cohort was small, with a mean estimated body of 57 kilogram (95% CI, 55.5–58.7 kg), resulting in a lower dose of midazolam needed for adequate sedation. Nevertheless, the dose per kilogram per day of midazolam given was also small; this could be due to the co-administration of multiple therapeutic agents with synergistic effect. Moreover, there were about 45% of patients in our series had their symptom control time less than 80% of the sedated period, suggesting that there could be room for titrating the medication to attain a satisfactory symptom control.
As suggested in some guidelines, in case the patient developed agitated delirium, antipsychotics like haloperidol should be the drug of choice as midazolam single agent may precipitate delirium (11). Delirium is one of the most frequent complications encountered in palliative care with prevalence between 13% and 88% and is typically irreversible during terminal phase (17). Several meta-analyses show that antipsychotics are useful for the treatment of delirium. Among the typical antipsychotics, haloperidol is one of the most common drugs used, with randomized clinical trials showing its benefit in improving delirium symptoms in palliative care setting. It also has other therapeutic effects including antiemetics and sedating effects which may be beneficial in managing symptoms during the end of life (18). Common dose for haloperidol suggested for controlling delirium would be 0.5 to 10 milligram per day by oral, intravenous or subcutaneously route. Again, the median dose used in our case series was 4 milligrams (range, 1–5 mg), which was lower than the suggested dose. Although in our case series showed that the addition of other therapeutic agents was not associated with increased rate of successful symptom control with sedation, it could lower the dose of the therapeutic agents to achieve a similar effect and avoid side effects associated with high dose.
In our cohort, deranged liver function was associated with better symptom control. It could be related to both the pharmacology of midazolam and haloperidol, and patients’ factor. Midazolam is eliminated by hepatic metabolism by cytochrome P450-3A4 to hydroxylated metabolites which are then excreted in urine. Clearance of midazolam is reduced in association with liver impairment and the mean half-life of midazolam is increased (19). Similarly, haloperidol is also metabolized by the P450 system (20), though the effect of impaired liver function on the metabolism of haloperidol is less extensively studied in literatures. Therefore, with a similar dose of midazolam and haloperidol, the therapeutic effect would be more pronounced in patients with liver impairment. Also, inpatient with impending liver failure, their sensorium would be decreased due to the presence of hepatic encephalopathy, and would also be contributed to a deeper sedated level compared with patients with normal organ function.
Sedation score and monitoring during sedation
During palliative sedation, multiple areas should be monitored including symptomatic relief, depth of sedation and the side effects of sedation. In our unit, the clinical symptoms of patients were documented in our end of life care plan template, with multiple symptoms would be assessed during nursing routine and oncologist ward round to ensure adequate symptoms control. However, the depth of sedation was assessed by modified POSS without addressing the symptoms of agitation, which was common in palliative care setting. When patients were recruited into the ECP, conscious level was assessed with a semi-quantitative level, and is less objective when compared with some widely validated tools, e.g., The Richmond Agitation-Sedation Scale modified for palliative care inpatients (RASS-PAL) (21).
As the depth of sedation was not objectively assessed, titration of sedating drugs would be less optimal as physicians in charge may be more conservative in titrating up the dose of medication needed for adequate sedation. In some studies, underuse of medicines was not uncommon and was mainly due to a lack of knowledge, unwarranted beliefs, avoiding the perception of giving “excessive” doses, and even because of fear among caregivers of being accused of “killing” the patient. Use of objective scale can promote good clinical documentation and communication between staff, and to serve as a guide for drug escalation to avoid undertreatment of symptoms. Deschepper et al. proposed the use of an integrated mixed method to improve the evaluation of palliative sedation which includes observational scales, subjective assessments reported by caregivers and family, and electrophysiological (EP) techniques (22). Though the feasibility of such an approach seems remote especially concerning about the use of EP techniques, our current symptoms assessment in ECP template, which includes symptom assessment by caregivers and observation by healthcare workers, attempts to address the need for monitoring of symptoms to ensure patient’s comfort during their last days of life.
Procedure on obtaining consent from patient and relatives for palliative sedation
In our case series, only 8.6% of our patients had advance directive available before the admission to palliative care ward, and discussion of palliative done was only done in an additional 21% of patients before the commencement of treatment. However, almost all relatives were informed of the decision on sedation and confirmed the decision for “do not attempt resuscitation” on their loved ones. In traditional Chinese culture, death was a taboo and mentioning it was disrespectful (23). With the heavy influence by Confucianism, family and society are held in higher regard than the individual, and it is not uncommon to leave end-of-life decisions to the family members, which is very different from western culture (24). The situation in our department concurred with this phenomenon. One possible explanation was that the discussion only held when patients were not conscious enough to decide for palliative sedation. Previously, advance directive was deemed feasible in Chinese terminal cancer patients in out-patient setting, especially if patients had insight about their poor prognosis and there was no family objection during the discussion (25). Therefore, earlier discussion on advance directive should be encouraged. Details of advance care planning should include the possibility of palliative sedation particularly in patients who are having a high chance in need of palliative sedation, e.g., patient with extensive lung metastases or primary central nervous system tumors.
The details of discussion concerning the use of palliative sedation could be inadequately assessed due to the retrospective nature of our study. However, though all patients had been assessed for possible reversible causes of severe distress and with treatments tailored accordingly, these might not be adequately conveyed to the relatives, as evidenced by withdrawal of sedation in one of our patients due to relatives’ request. In the ESMO guideline on palliative sedation in the end of life, they suggested the discussion of palliative should include the followings (11):
- the patient’s general condition and the cause of the distress;
- acknowledgment that prior treatments have not been successful;
- current prognosis, including predictions about survival;
- rationale, aims, and methods available for the use of palliative sedation, including the depth of planned sedation, patient monitoring, and, if appropriate, the possibility of planned;
- weaning and even discontinuation of sedation;
- alternative treatment options, the likelihood that they may relieve distress, and the expected survival associated with each.
Frequent communication with caregivers is required to enhance their understanding and is crucial for loved ones to overcome the grieving process after the patients’ death.
Weakness of study
The retrospective nature of the study had some limitations. Firstly, only standard symptom documentation was only available in patients enrolled in the ECP. In those who were not recruited, especially during the initial phase of light sedation while patients’ consciousness remains, the symptom documented could only be retrieved in hospital notes which might not be entirely comprehensive. Secondly, generalization was also difficult as the study was conducted on terminal cancer patients within an oncology unit, which may not represent all terminal patients in other centers or with different etiologies. Thirdly, the sedation score we used were not using validated scales like RASS-PAL, which might be difficult to compare with other publications.
Future direction
First, guidelines and consensus on palliative sedation should be developed across the territory to minimize variation in the practice of palliative sedation within Hong Kong. A useful example would be from Calgary, Canada on how they developed their clinical practice guideline in 2003. They recommended multiple steps which include choosing a workable definition of palliative sedation within the region; developing criteria for the use of palliative sedation; and identifying the actions to be undertaken before the initiation of palliative sedation (26). After establishment of a workable consensus, further studies involving various institutions could be conducted to strengthen our clinical evidence in this area in the future.
Second, public awareness on advance directives and advance care plans should also be enhanced. Education on the use of palliative sedation should be provided to health care workers that involve in end-of-life care, as well as to the general public to clarify misunderstandings and ethical concerns on the use of palliative sedation. Early discussion among physicians, patients and relatives should be undertaken regarding the end of life treatment preferences and need for palliative sedation before clinical deterioration (27).
Conclusions
Use of palliative sedation is safe and effective in managing refractory symptoms especially towards the end of life as demonstrated in our study and is not associated with worsening of survival. Deranged liver function was associated with better symptom control. The dose of midazolam and haloperidol needed for adequate symptom control were lower than suggested dose in Western guidelines. Further studies on the dose requirement in Chinese population in other palliative units are warranted to establish dosage guidance. Establishing consensus and guidelines on palliative sedation in Hong Kong should be the way forward to minimize variation in practice and to ensure quality care to this group of patients.
Acknowledgments
Funding: The statistical analysis was supported by Assistant Professor Dr. TC Lam, Department of Clinical Oncology, the University of Hong Kong, and statistician Mr. JJ Hwang, MSc (Statistics).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rebecca Yeung and Tai Chung Lam) for the series “Integrating Palliative Medicine in Oncology Care: The Hong Kong Experience” published in Annals of Palliative Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm.2019.09.05). The series “Integrating Palliative Medicine in Oncology Care: The Hong Kong Experience” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by New Territories West Cluster Research Ethics Committee (Ref no. NTWC/REC/19026). Individual informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cherny NI, Radbruch L. Board of the European Association for Palliative Care. European Association for Palliative Care (EAPC) recommended framework for the use of sedation in palliative care. Palliat Med 2009;23:581-93. [Crossref] [PubMed]
- Verkerk M, van Wijlick E, Legemaate J, et al. A National Guideline for Palliative Sedation in The Netherlands. J Pain Symptom Manage 2007;34:666-70. [Crossref] [PubMed]
- Abarshi E, Rietjens J, Robijn L, et al. International variations in clinical practice guidelines for palliative sedation: a systematic review. BMJ Support Palliat Care 2017;7:223-9. [Crossref] [PubMed]
- Maltoni M, Scarpi E, Nanni O. Palliative sedation for intolerable suffering. Curr Opin Oncol 2014;26:389-94. [Crossref] [PubMed]
- Kirk TW, Mahon MM. National Hospice and Palliative Care Organization (NHPCO) Position Statement and Commentary on the Use of Palliative Sedation in Imminently Dying Terminally Ill Patients. J Pain Symptom Manage 2010;39:914-23. [Crossref] [PubMed]
- Maltoni M, Pittureri C, Scarpi E, et al. Palliative sedation therapy does not hasten death: results from a prospective multicenter study. Ann Oncol 2009;20:1163-9. [Crossref] [PubMed]
- Beller EM, van Driel ML, McGregor L, et al. Palliative pharmacological sedation for terminally ill adults. Cochrane Database Syst Rev 2015;1:CD010206. [Crossref] [PubMed]
- Lo SH, Chan CY, Chan CH, et al. The implementation of an end-of-life integrated care pathway in a Chinese population. Int J Palliat Nurs 2009;15:384-8. [Crossref] [PubMed]
- Tin WY, Hwang LM, Lo SH, et al. Implementation of End-of-Life Integrated Care Pathway in Hong Kong – Outcome Audit & Relatives’ Satisfaction Survey. Asia Pacific Hospice Conference 2015, Oral Presentation.
- Pecking M, Montestruc F, Marquet P, et al. Absolute bioavailability of midazolam after subcutaneous administration to healthy volunteers. Br J Clin Pharmacol 2002;54:357-62. [Crossref] [PubMed]
- Cherny NI. ESMO Guidelines Working Group. ESMO Clinical Practice Guidelines for the management of refractory symptoms at the end of life and the use of palliative sedation. Ann Oncol 2014;25 Suppl 3:iii143-52. [Crossref] [PubMed]
- Parra Palacio S, Giraldo Hoyos CE, Arias Rodríguez C, et al. Palliative sedation in advanced cancer patients hospitalized in a specialized palliative care unit. Support Care Cancer 2018;26:3173-80. [Crossref] [PubMed]
- Gu X, Cheng W, Chen M, et al. Palliative sedation for terminally ill cancer patients in a tertiary cancer center in Shanghai, China. BMC Palliat Care 2015;14:5. [Crossref] [PubMed]
- Radha Krishna LK, Poulose VJ, Goh C. The use of midazolam and haloperidol in cancer patients at the end of life. Singapore Med J 2012;53:62-6. [PubMed]
- Chiu TY, Hu WY, Lue BH, et al. Sedation for refractory symptoms of terminal cancer patients in Taiwan. J Pain Symptom Manage 2001;21:467-72. [Crossref] [PubMed]
- Stiel S, Nurnus M, Ostgathe C, et al. Palliative sedation in Germany: factors and treatment practices associated with different sedation rate estimates in palliative and hospice care services. BMC Palliat Care 2018;17:48. [Crossref] [PubMed]
- Grassi L, Caraceni A, Mitchell AJ, et al. Management of Delirium in Palliative Care: a Review. Curr Psychiatry Rep 2015;17:550. [Crossref] [PubMed]
- Lindqvist O, Lundquist G, Dickman A, et al. Four essential drugs needed for quality care of the dying: a Delphi-study based international expert consensus opinion. J Palliat Med 2013;16:38-43. [Crossref] [PubMed]
- Midazolam FDA drug insert. Accessed online on 21st February 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208878Orig1s000lbl.pdf
- Haloperidol FDA drug insert. Accessed online on 21st February 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/015923s092lbl.pdf
- Bush SH, Grassau PA, Yarmo MN, et al. The Richmond Agitation-Sedation Scale modified for palliative care inpatients (RASS-PAL): a pilot study exploring validity and feasibility in clinical practice. BMC Palliat Care 2014;13:17. [Crossref] [PubMed]
- Deschepper R, Laureys S, Hachimi-Idrissi S, et al. Palliative sedation: Why we should be more concerned about the risks that patients experience an uncomfortable death. Pain 2013;154:1505-8. [Crossref] [PubMed]
- Chan CL, Chow AY. Death, dying and bereavement: the Hong Kong Chinese experience. Hong Kong: Hong Kong University Press, 2006.
- Lee MC, Hinderer KA, Kehl KA. A Systematic Review of Advance Directives and Advance Care Planning in Chinese People. From Eastern and Western Cultures. J Hosp Palliat Nurs 2014;16:75-85. [Crossref]
- Wong SY, Lo SH, Chan CH, et al. Is it feasible to discuss an advance directive with a Chinese patient with advanced malignancy? A prospective cohort study. Hong Kong Med J 2012;18:178-85. [PubMed]
- Braun TC, Hagen NA, Clark T. Development of a clinical practice guideline for palliative sedation. J Palliat Med 2003;6:345-50. [Crossref] [PubMed]
- Cheng HW. Advance Care Planning in Chinese Seniors: Cultural Perspectives. J Palliat Care 2018;33:242-6. [Crossref] [PubMed]


