Health-related quality of life across cancer cachexia stages
Introduction
Up to 80% of advanced cancer patients will experience cachexia in their disease trajectory (1,2). It is known that cancer cachexia (CC) has a negative effect on function, treatment tolerance and overall mortality, with cachexia being the cause of death in 30% of cancer patients (3). As such, understanding the effect of CC on health-related quality of life (HRQOL) is important. HRQOL is a multidimensional concept including, but not limited to, symptoms of disease, side effects of treatment, perception of wellbeing and life satisfaction and measures of physical, mental and social function (4). Significant associations have been identified between weight loss, malnutrition, CC and poor HRQOL outcomes (5-7). This paper will review the current definition and methods to classify CC. Tools used to measure HRQOL in cachexia will be identified. Additionally, results from our laboratory assessing HRQOL along the CC continuum and the factors driving poor HRQOL in CC will be presented. Finally, preliminary evidence for the use of cannabinoids to relieve symptoms that impair HRQOL will be put forth.
CC: definition and classification
In 2011, Fearon et al. published the following international consensus statement defining CC, “A multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. The pathophysiology is characterized by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism” (8). Furthermore, criteria for diagnosis were put forth, which included: (I) weight loss >5% in 6 months (in absence of starvation) or, (II) BMI <20 and any degree of weight loss >2% or, (III) appendicular skeletal muscle index <7.26 kg/m2 in males or <5.45 kg/m2 in females with weight loss of >2% (8). Once cachexia is diagnosed, Fearon et al. proposed a classification system dividing cachexia into three stages: pre-cachexia (PC), cachexia (C) and refractory cachexia (RC). PC is defined as a ≤5% weight loss with anorexia and metabolic change. C patients present with weight loss of >5%, or BMI <20 and weight loss of >2%, or sarcopenia and weight loss of >2%. They also often have reduced food intake and systemic inflammation. In RC, the cancer is pro-catabolic and not responsive to treatment. Additionally, patients will have low performance scores.
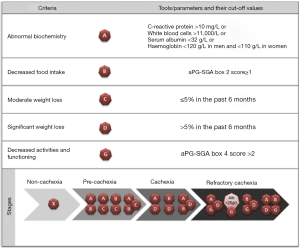
Following this very important work, Vigano et al. established a CC classification system that uses clinically available tools (Figure 1) (9). Classification is based on five criterion that can be determined using the results of a simple blood test and the abridged Patient-generated Subjective Global Assessment (aPG-SGA) questionnaire. Using these criteria, PC is classified as a combination of abnormal biochemistry with decreased food intake or moderate weight loss, or decreased food intake with moderate weight loss. C is identified by a severe weight loss with either abnormal biochemistry or decreased food intake. RC is classified as C with decreased activities and function, or albumin <20 g/L with decreased activities and function.
Methods to assess HRQOL in CC patients
Tools for assessing HRQOL in CC are limited. In a 2013 review, Wheelwright et al. identified the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) as the only cachexia-specific instrument available at the time (10). The FAACT tool assesses five domains: physical wellbeing, social/family wellbeing, emotional wellbeing, functional wellbeing and CC specific symptoms. However, weaknesses in the methodology used to validate the tool, the absence of additional psychosocial domains affecting patients with CC and doubt in the ability to use the tool internationally led the authors to conclude that a robust instrument to assess HRQOL in CC is lacking (10).
Since then, the European Organization for Research and Treatment of Cancer (EORTC)-CAX24 scale has been developed to fill this void (11). To be used with the more generic HRQOL assessment tool, the EORTC-QLQ-C30 (12), it proposes five domains and four individual items capturing relevant issues affecting CC patients. These include: food aversions (5 questions), eating and weight loss worry (3 questions), eating difficulties (3 questions), loss of control (6 questions), physical decline (3 questions) and dry mouth, indigestion/heartburn, forcing self to eat and inadequate information. This tool is currently in the process of being fully validated on an international scale (11).
A recent study by Zhou et al. used the Chinese version of the MD Anderson Symptom Inventory (13), with the addition of 8 cachexia-specific symptoms (feeling dizzy, early satiety, lack of energy, changes in taste and smell, diarrhea, constipation, anxiety, and depression), to assess symptom burden among the CC stages (14). Results suggested that lack of appetite was the most frequent and severe symptom among the four CC groups, followed by fatigue, disturbed sleep, lack of energy and distress. The authors cite limitations of this study, which include (I) the lack of validation of the new tool developed; and (II) the CC staging method used, based on the work of Blum et al. (7), which the authors criticize as not using sarcopenia as part of their classification system, and only weight loss as the definition for RC (14).
Non-cachexia specific tools to assess HRQOL in CC
Due to the paucity of CC specific instruments to assess HRQOL, surrogates must be identified. Ideally, tools would be simple to use and not burdensome to patients. There has been some work pursuing correlations between “feeling of wellbeing” (FWB) as a single item on a questionnaire, and total scores on multi-item HRQOL assessment instruments. Stiel et al. (15) analyzed the relationship between the “How do you feel today?” question on the German Minimal Documentation System (MIDOS) (16) and total scores of the EORTC-QLQ-C30 and the Functional Assessment of Cancer Therapy-General (FACT-G) (17). In both instances, social domains were not captured by the single question. However, it was significantly associated with the physical (r=0.38, P<0.01), cognitive (r=0.34, P<0.01), emotional (r=0.33, P<0.01) and role functioning (r=0.26, P<0.05) domains of the EORTC-QLQ-C30 and the physical (r=0.58, P<0.01), functional (r=0.42, P<0.01) and emotional (r=0.38, P<0.01) domains of the FACT-G (15). Similarly, Bush et al. found a moderate association between the FWB question on the Edmonton Symptom Assessment System (ESAS) (18) and total FACT-G score (r=0.48, P<0.0001) (19). In a smaller study, Paiva et al. compared ESAS FWB and the EORTC-QLQ-C30, with a moderate association with the overall symptom scales (r=0.61, P<0.0001) (20).
Another single item that may prove useful in identifying poor HRQOL is the Distress Thermometer (DT) (21). The DT is a vertical scale ranging from 0 to 10 asking patients to rate their feeling of distress in the past week, with zero denoting “no distress” and ten indicating “extreme distress.” The National Comprehensive Cancer Network defined distress as, “a multifactorial, unpleasant, emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment. Distress extends along a continuum, ranging from common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis” (22). High levels of distress are associated with poor effect on quality of life (23). The DT is comparable to longer screening tools in its ability to correctly identify distress among cancer patients; a cutoff of four has been associated with the best sensitivity and specificity (24). To the knowledge of the authors, there is no specific tool identifying distress among CC patients.
Assessing HRQOL in CC using single-item measures: original research
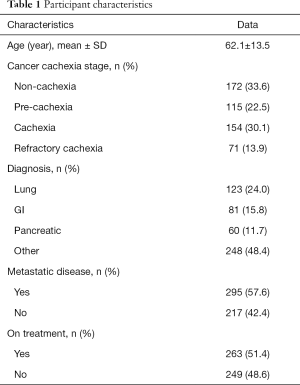
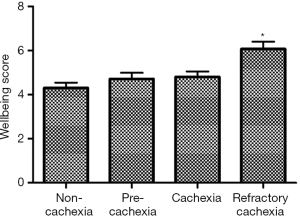
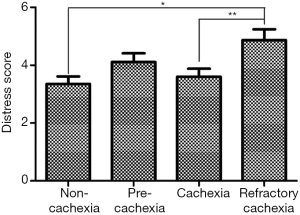
Given the current lack of a validated CC-specific HRQOL assessment tool, we decided to retrospectively examine how the single-item of FWB from the ESAS questionnaire and the DT would differ between the CC stages. Five hundred and twelve patients who were referred to the Cancer Rehabilitation Program of the McGill University Health Centre (Montreal, Canada), completed these two questionnaires and were separated into CC stages, as per the classification system of Vigano et al. (9). Participant characteristics are reported in Table 1. Mixed model ANOVA with post hoc Tukey adjustment was used to identify differences in wellbeing and distress between CC groups. The models controlled for age, sex, diagnosis, current treatment and the presence of metastatic disease. Significance was determined at P<0.05. Figures 2 and 3 illustrate the results. RC patients had a significantly greater poor sense of wellbeing than the other cachexia stages (RC: 6.07±0.33) (Figure 2). Significant differences in distress were identified between RC patients and those with NC and C, but not with PC (RC: 4.87±0.38, NC: 3.35±0.26, PC: 4.11±0.30, C: 3.60±0.28) (Figure 3).
Full table
With the data suggesting differences between CC stages and the HRQOL-surrogate measurements of FWB and distress, we then wanted to understand what is driving these differences. To achieve this, a robust regression model was created with either distress or FWB as the outcome measure, which was dependent on the other measures in ESAS, namely pain, tiredness, nausea, depression, anxiety, drowsiness, appetite and shortness of breath (SOB). Additionally, age and sex were considered in the model. Results for each are shown in Tables 2 and 3. FWB is negatively affected by worsening appetite in all CC stages except NC. This mirrors results from Zhou et al. (14). Additionally, anxiety had a poor effect on FWB in all CC stages. Fatigue was also predictive of poor wellbeing in the C and RC stages. Feelings of distress increased in all CC stages, except RC, as anxiety increased. The relationship between distress and anxiety has previously been demonstrated in ambulatory cancer patients (21). None of the ESAS symptoms were significantly related to feelings of distress in RC patients.

Full table

Full table
Symptom of interest: anorexia
The presence of anorexia leads to decreased food intake, which is a characteristic of cachexia; in our laboratory’s previous work creating a CC staging system, 63% of patients reported decreased intake, reflecting a lack of appetite (9). While the cluster of other CC symptoms such as anxiety, fatigue, pain and depression have effective pharmacological and nonpharmacological interventions available, the ability to treat anorexia remains difficult. Orexigenic agents used to reverse anorexia include corticosteroids, megestrol acetate, serotonin antagonists, anamorelin (ghrelin-mimetic) and cannabinoids.
Corticosteroids
Corticosteroids have been used effectively in the treatment of many symptoms in advanced disease. Improvements have been demonstrated in anorexia, but also in relieving symptoms of pain, fatigue, chemotherapy-induced nausea and vomiting and overall quality of life (25). Unfortunately, the reversal of anorexia using corticosteroids is short lived, generally lasting less than 4 weeks (26). Additionally, long-term use of corticosteroids is associated with myopathy, gluconeogenesis leading to insulin resistance, immunosuppression, bone loss and mood disturbances (25).
Megestrol acetate
A recent updated Cochrane Review on the effectiveness of megestrol acetate for the reversal anorexia in cancer patients demonstrated favorable results (27). Megestrol acetate was effective in significantly improving both appetite when compared to placebo [RR 2.19 (1.4–3.4)] (27). Modest weight gain was also observed: 1.96 kg (95% CI: 1.11–2.81 kg) (27). Despite this, the quality of evidence for the improvement of anorexia versus placebo was graded as “very low” due to possible bias introduced from unclear blinding methods, sequence generation and allocation concealment. Additionally, side-effects such as edema, dyspnea, thromboembolic events and death were associated with the use of megestrol acetate versus placebo in both low and high doses (±800 mg/day) (27).
Serotonin antagonist: cyproheptadine
The use of cyproheptadine as an orexigenic agent for advanced cancer patients has yielded few benefits. Kardinal et al. only demonstrated a moderate improvement in appetite over placebo, with weight loss in both groups (4.5±0.72 versus 4.95±1.01 lb, P=0.72) (28).
Ghrelin mimetic: anamorelin
Recent phase III trials have demonstrated a positive effect of anamorelin on both appetite and weight vs placebo in stage III/IV non-small cell lung cancer patients. In the ROMANA 1 and ROMANA 2 studies, participants were given 100 mg anamorelin/day or placebo for 12 weeks (29). Pooled analysis of the studies demonstrated the anamorelin group had modest increases in mean total body weight (ROMANA 1 anamorelin: 2.2±0.33 kg, placebo: 0.14±0.36 kg; ROMANA 2 anamorelin: 0.95±0.39 kg, placebo: −0.57±0.44 kg) and median lean body mass (ROMANA 1 anamorelin: 0.99 kg (95% CI: 0.61 to 1.36 kg), placebo: −0.47 kg (95% CI: −1.00 to 0.21 kg); ROMANA 2 anamorelin: 0.65 kg (95% CI: 0.38 to 0.91 kg), placebo: −0.98 (95% CI: −1.49 to −0.41 kg) (29). Strength, as measured by handgrip dynamometry, was not significantly improved. Overall mean anorexia-cachexia scale score, as measured by FAACT, was significantly greater in the anamorelin group (29). There were no differences in treatment-related adverse events between study groups; the most common were hyperglycemia, nausea and edema (29). While its effect in treating anorexia seems promising, anamorelin is not yet commercially available.
Cannabinoids
The potential effect of cannabinoids on appetite and weight has been repeatedly reviewed in patients with cancer and HIV/AIDS (30-33). Two studies looked at natural extracts, six studies looked at dronabinol, a synthetic cannabinoid, as orexigenic agents and one study assessed nabilone. In 2006, the Cannabis-In-Cachexia-Study-Group compared the effects of cannabis extract, delta-9-tetrahydrocannabinol (THC), and placebo on appetite and quality of life in patients with cancer-related anorexia-cachexia syndrome (34). The cannabis extract, administered at a dose of 2.5 mg of THC and 1 mg of cannabidiol (CBD), was well tolerated by patients with anorexia. However, no significant differences in appetite and HRQOL were found for cannabis extract as compared to placebo (34). In another study, higher doses of natural cannabinoids (up to 22.5 mg/day of THC) provided more consistent and favorable results for appetite stimulation and decreased weight loss associated with cancer (total weight gain of 1.25 lb; on placebo: total weight loss of 21.25 lb) (30). Equally, the combination of both oral and inhaled methods of administration provided favorable results for an increase and stabilization of weight in HIV patients (30). Studies that examined dronabinol also found limited and low-quality evidence supporting cannabinoids for appetite stimulation and weight gain in cancer patients. More recently, a randomized, double-blind, placebo-controlled study evaluated the effect of nabilone (0.5 mg/day/2 weeks followed by 1.0 mg/day/6 weeks) in patients with advanced non-small cell lung cancer. Patients on nabilone (n=9) showed an increase in their average caloric intake (342 kcal/day) and significant improvements in their quality of life particularly for role functioning, emotional functioning, social functioning, pain, and insomnia, which were not seen in the patients on placebo (n=13) (35).
Assessing cannabinoids for increasing appetite and stabilizing weight in chronic cancer and non-cancer diseases: original research
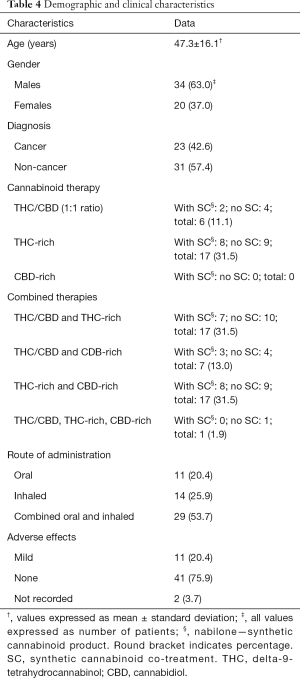
In order to gather more specific data on the effect of different types of cannabinoids on appetite and weight in chronic cancer and non-cancer diseases, a retrospective chart review was conducted at Santé Cannabis, the only community-based, physician-lead, medical cannabis clinic in Quebec, Canada. At baseline, 54 patients with “increase appetite” as a treatment goal completed the ESAS question on appetite, with 51 subjects also having their weight measured. These assessments were repeated at 3-month follow-up. The mean age of patients was 47.3±16.1 years; 63% were male and 43% of our sample was represented by patients with a cancer diagnosis (Table 4).
Full table
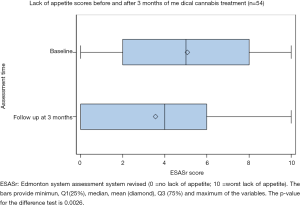
Of the 54 patients analyzed, the ESAS score for lack of appetite significantly improved between baseline (5.07±3.21) and follow-up (3.56±3.15, paired t-test P=0.0026) (Figure 4). Bivariate regression reveals a significant improvement with the use of nabilone (−2.73, 95% CI: −4.19 to −1.27, P=0.0358). Route of administration also had an effect on appetite: (I) favoring only inhaled vs. only oral (−2.36, 95% CI: −4.17 to −0.54, P=0.024) and (II) favoring combined oral and inhaled vs. only oral (−2.00, 95% CI: −3.26 to −0.74, P=0.023). With regression models adjusted for age and gender (multivariate), only a marginal improvement was detected for the use of nabilone (−2.84, 95% CI: −4.34 to −1.34, P=0.0521). A more pronounced improvement was demonstrated among the methods of administration: (I) favoring only inhaled vs. only oral (−2.01 to 95% CI: −4.85 to −1.17, P=0.006) and (II) favoring combined oral and inhaled vs. only oral (−2.34 to 95% CI: −3.61 to −1.07, P=0.009).
Among the 51 subjects who were examined for weight change over time, there was no significant difference found and weight remained stable between baseline (70.7±14.6 kg) and 3-month follow-up (71.0±14.8 kg). Regression models, with and without adjustment for age and gender, did not show any difference in weight associated with nabilone use or with different routes of administration.
The majority of study patients did not report any side effects to cannabinoids (Table 4). Eleven patients reported mild side effects, including anxiety, fatigue, dizziness and dry mouth.
Conclusions
Despite the incidence and prevalence of CC, there is still a paucity of data regarding its impact on HRQOL. Latest research in this area has focused on developing and/or applying routinely available criteria to identify CC stages in clinical practice, specific multidimensional tools (such as FAACT or EORTC-CAX24) or non-specific single-item scales (such as DT and FWB scale from ESAS) to assess HRQOL across CC stages and orexigenic agents such as anamorelin and cannabinoids. Original research from our group suggests wellbeing is negatively affected by anorexia and anxiety in all CC stages, with fatigue also being predictive of poor wellbeing in the cachexia and RC stages. Cannabinoids, when prescribed through an interdisciplinary, physician-lead, care model appear to be promising orexigenic agents in chronic cancer and non-cancer diseases, particularly if used concomitantly through the oral and the inhalation route of administration. Future research should further validate both multidimensional and single-item tools to measure HRQOL in patients at different stages of CC, for whom the above pharmacological interventions are trialed to improve appetite and stabilize weight.
Acknowledgements
The authors would like to extend their gratitude to the staff and patients of both the MUHC Cancer Rehabilitation Program and Santé Cannabis. P Kasvis would like to thank the Cedars Cancer Foundation at the MUHC for the Henry R. Shibata Fellowship awarded to her, allowing for the undertaking of this project. P Kasvis and A Vigano wish to also acknowledge Dr. Leonard Rosenthall, who provided much guidance with the statistical analysis of this project.
Footnote
Conflicts of Interest: A Vigano is the Research Director of Santé Cannabis—a medical cannabis clinic specializing in clinical research; the principal investigator for a phase II and a phase III clinical trial sponsored by Tetra Bio-Pharma, Inc. The other authors have no conflicts of interest to declare.
References
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153-66. [Crossref] [PubMed]
- Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016;5:e200. [Crossref] [PubMed]
- Melstrom LG, Melstrom K Jr, Ding X, et al. Mechanisms of skeletal muscle degradation and its therapy in cancer cachexia. Histol Histopathol 2007;22:805-14. [PubMed]
- Cella D, Stone AA. Health-related quality of life measurement in oncology: Advances and opportunities. Am Psychol 2015;70:175. [Crossref] [PubMed]
- Nourissat A, Vasson M, Merrouche Y, et al. Relationship between nutritional status and quality of life in patients with cancer. Eur J Cancer 2008;44:1238-42. [Crossref] [PubMed]
- Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Ann Oncol 2014;25:1492-9. [Crossref] [PubMed]
- Blum D, Omlin A, Baracos VE, et al. Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol 2011;80:114-44. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Vigano AA, Morais JA, Ciutto L, et al. Use of routinely available clinical, nutritional, and functional criteria to classify cachexia in advanced cancer patients. Clin Nutr 2017;36:1378-90. [Crossref] [PubMed]
- Wheelwright S, Darlington AS, Hopkinson JB, et al. A systematic review of health-related quality of life instruments in patients with cancer cachexia. Support Care Cancer 2013;21:2625-36. [Crossref] [PubMed]
- Wheelwright SJ, Hopkinson JB, Darlington A-S, et al. Development of the EORTC QLQ-CAX24, a questionnaire for cancer patients with cachexia. J Pain Symptom Manage 2017;53:232-42. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Wang XS, Wang Y, Guo H, et al. Chinese version of the MD Anderson symptom inventory. Cancer 2004;101:1890-901. [Crossref] [PubMed]
- Zhou T, Yang K, Thapa S, et al. Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage 2017;53:919-26. [Crossref] [PubMed]
- Stiel S, Psych D, Kues K, et al. Assessment of quality of life in patients receiving palliative care: comparison of measurement tools and single item on subjective well-being. J Palliat Med 2011;14:599-606. [Crossref] [PubMed]
- Stiel S, Matthes M, Bertram L, et al. Validation of the new version of the minimal documentation system (MIDOS) for patients in palliative care: the German version of the Edmonton symptom assessment scale (ESAS). Schmerz 2010;24:596-604. [Crossref] [PubMed]
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [PubMed]
- Bush SH, Parsons HA, Palmer JL, et al. Single-vs. multiple-item instruments in the assessment of quality of life in patients with advanced cancer. J Pain Symptom Manage 2010;39:564-71. [Crossref] [PubMed]
- Paiva CE, Paiva BSR. Searching for a simple assessment tool capable of estimating quality of life in palliative care clinical practice: is a feeling of well-being a good candidate tool as a single item? J Palliat Med 2011;14:1281-82. [Crossref] [PubMed]
- Patrick-Miller LJ, Broccoli TL, Much JK, et al. Validation of the Distress Thermometer: A single item screen to detect clinically significant psychological distress in ambulatory oncology patients. J Clin Oncol 2004;22:6024. [Crossref]
- Holland JC, Andersen B, Breitbart WS, et al. Distress management. J Natl Compr Canc Netw 2010;8:448-85. [Crossref] [PubMed]
- Zabora J, Brintzenhofeszoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology 2001;10:19-28. [Crossref] [PubMed]
- Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer 2005;103:1494-502. [Crossref] [PubMed]
- Yennurajalingam S, Bruera E. Role of corticosteroids for fatigue in advanced incurable cancer: is it a ‘wonder drug’or ‘deal with the devil’. Curr Opin Support Palliat Care 2014;8:346-51. [Crossref] [PubMed]
- Mattox TW. Cancer Cachexia: Cause, Diagnosis, and Treatment. Nutr Clin Pract 2017;32:599-606. [Crossref] [PubMed]
- Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, et al. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 2013.CD004310. [PubMed]
- Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer 1990;65:2657-62. [Crossref] [PubMed]
- Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016;17:519-31. [Crossref] [PubMed]
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol 2006;105:1-25. [Crossref] [PubMed]
- Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015;313:2456-73. [Crossref] [PubMed]
- Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol 2016;23:S8. [PubMed]
- Turgeman I, Bar-Sela G. Cannabis use in palliative oncology: A review of the evidence for popular indications. Isr Med Assoc J 2017;19:85-8. [PubMed]
- Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 2006;24:3394-400. [Crossref] [PubMed]
- Turcott JG, Núñez M, Flores-Estrada D, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. Support Care Cancer 2018;26:3029-38. [Crossref] [PubMed]