
Combination therapy in cachexia
Introduction
Ken Fearon recognized that the defining features of cachexia in humans (weight loss, reduced food intake, and chronic inflammation) might provide a framework for classification of cachexia and a rationale for identifying multiple therapeutic targets (1). By combining pharmacological and non-pharmacological interventions, the multifaceted mechanisms of this complex syndrome could be addressed simultaneously, resulting in improved protein and caloric intake, gains in muscle and fat, and better physical function. Furthermore, the role of co-morbid conditions such as age-related sarcopenia and immobility in contributing to muscle-wasting was appreciated, providing justification for including physical activity and nutritional support in formulating comprehensive cachexia therapy for the “whole patient”. Pre-clinical research elucidating the complex molecular mechanisms of cancer cachexia revealed additional support for the inclusion of specific individual interventions into multimodality treatment strategies and increased susceptibility for cachexia because of genetic variation.
However, for more than two decades, despite recurrent glimpses of success, strong evidence for this concept of “multimodal therapy for a multidimensional problem” has proven to be elusive. This review will highlight research that provides support for using a variable combination of nutritional, exercise, and pharmacological interventions. The review also focuses on past clinical trials incorporating nonsteroidal anti-inflammatory drugs (NSAIDs) and omega-3 supplements in combination therapy—two of the principle agents, along with supportive care, that are included in MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia), a multi-center trial developed by the late Ken Fearon (2).
A theoretical model of multimodal therapy for cachexia is shown in Figure 1. Although there may be debate about the composition or relative importance of specific individual interventions, the model illustrates the rationale for multimodality treatment directed at the various cachexia mechanisms.
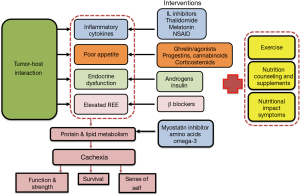
An individualized multimodal model
Based on patient preference
The treatment goal for many patients with cachexia is to maintain physical function and independence. In addition, fatigue often occurs in patients with cachexia along with muscle weakness and impaired ability to perform activities of daily living (ADLs) (3). For some individuals, the psychosocial aspects of the cachexia/anorexia syndrome (CAS), such as altered body image and the inability to enjoy meals with family members, may carry greater importance. A systematic review identified five areas of health-related quality of life (HRQoL) themes based on patient quotes extracted from the literature (4). Loss of eating as pleasure, loss of independence, and physical decline were recognized as important components along with psychosocial themes such as relationships, coping, and knowledge of the condition. A cancer cachexia-specific questionnaire, the QLQ-CAX24, has been developed in an attempt to be relevant, acceptable, and applicable to patients with cancer cachexia. The module was developed with the help of cancer patients at different stages of the cancer trajectory, from relatively soon after diagnosis to those approaching the end of life. The module contains five multi-item scales (food aversion, eating and weight-loss worry, eating difficulties, loss of control, and physical decline) and four single items (5). However, the QLQ-CAX24 is relatively new, with full validation to be assessed in a large international study. The only other available cancer cachexia-specific instrument, the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) (6), is part of the Functional Assessment of Chronic Illness Therapy measurement system FAACT has also been used to establish cut-off values (<37) for anorexia in patients starting chemotherapy (7),and is a single scale, whereas the QLQ-CAX24 provides a more comprehensive assessment and contains more items, including two scales (eating difficulties and physical decline). Although both FAACT and QLQ-CAX24 scales are specific to cancer cachexia and attempt to be all-inclusive, they do not necessarily capture an individual patient’s experience or needs.
Based on mechanisms of cachexia
Ideally, the treatment should also be modified to target the mechanisms affecting individual patients since the contribution of the different mechanisms of cachexia may vary. For example, not all patients have an elevated resting energy expenditure (REE) (some may be hypo- or eumetabolic), and for many with cancer cachexia, poor appetite is a common but not universal symptom.
The composition of a multimodal drug therapy could be modified depending on the proportional representation of the mechanisms of cachexia. Some of the components of the cachexia syndrome such as Nutritional Impact Symptoms (NIS) could be evaluated with tools such as the Edmonton Symptom Assessment Scale (ESAS) or Patient Generated Subjective Global Assessment (PG-SGA). Currently, laboratory tests such as C-reactive protein (CRP) and testosterone identify those patients with a pro-inflammatory state or low testosterone, while REE can be measured by indirect calorimetry (IC). The feasibility of using IC in daily practice may limit use of IC; however, centers with metabolic chambers or metabolic carts could take advantage of an accurate measure of REE. The emergence of brown fat as a potential driver of dysfunctional metabolism and inefficient energy production may increase the utility of IC. Specific interventions that are used in response to these markers include NSAIDs for elevated CRP >10 mg/L, beta-blockers for increased REE, and testosterone replacement for hypogonadal males. Note, however, that there is only preliminary evidence these medications are effective for cancer cachexia.
Additional clinical and biological markers are needed to better identify individuals who may respond to specific interventions. Biomarkers for cachexia are in their infancy but in future could facilitate earlier intervention, more effective individualized therapeutic regimens, and fewer unnecessary side effects. Pro-inflammatory cytokines, reactive oxygen species (ROS) (8,9), and single-nucleotide polymorphisms (10,11), are examples of markers that might aid in creating the biological profiles of cachectic patients.
Pharmacologic combinations
Monotherapy alone is unlikely to correct the multiple abnormalities associated with cachexia such as loss of muscle, fat, and physical function because of negative protein and energy balance driven by reduced food intake and metabolic dysfunction. Even an effective single pharmacological agent would have to be combined with dietary counseling, supplementation, symptom management, and exercise. Combination therapy should ideally have additive or even synergistic effects. Unfortunately, some current medications such as megestrol acetate (MA) or medroxyprogesterone may modulate one pathway favorably while encouraging other potentially harmful mechanisms. For example, although progestins can decrease pro-inflammatory cytokines and improve appetite, they are associated with endocrine abnormalities such as hypogonadism and hypoadrenalism (12). Other medications such as corticosteroids are effective appetite stimulants but have detrimental effects on muscle function, including myopathy. Some single interventions for cachexia under investigation, such as ghrelin, ghrelin agonists, beta blockers, and exercise, appear to be particularly promising in that they might affect more than one mechanism favorably, (e.g., inflammatory as well as neurohormonal pathways).
NSAIDs
Many NSAIDs, including celecoxib, ibuprofen, indomethacin, and etodolac (13), have been used alone or in combination with other agents for a variety of clinical outcomes related to cancer cachexia. A systematic literature review identified 11 of 13 trials either showing improvement or stabilization in weight or lean body mass (LBM) (14). However, the authors concluded the evidence was insufficient to recommend NSAIDs for cachexia outside clinical trials since seven were without a comparator, most had a small sample size, and a few were methodologically flawed. Trials using NSAIDs in combination with other agents included two trials combining NSAIDs with MA and NSAIDs with fish oil, which were compared with MA and fish oil alone, respectively.
A prospective randomized trial of ibuprofen (1,200 mg/day) in combination with MA, found significantly improved quality of life (QoL) and a median weight gain of 2.3 kg compared to a loss of 2.8 kg in patients on MA alone, with gastrointestinal (GI) cancer (15). Notably, individuals on combination therapy with ibuprofen did not appear to be at greater risk of major hemorrhage than those on MA alone (480 mg daily). A phase III trial in 104 advanced-stage gynecological cancer patients found MA plus L-carnitine, celecoxib, and antioxidants improved LBM as measured by dual energy X-ray absorptiometry (DEXA), REE, fatigue, and QoL compared to MA alone (16). Inflammation and oxidative stress measures including IL-6, TNF-α, CRP, and ROS decreased significantly with combination therapy.
A 6-week prospective study of 22 patients with advanced lung cancer compared celecoxib (200 mg twice daily) in combination with fish oil to fish oil alone (6 g daily in divided doses). Those receiving combination therapies had significantly lower CRP levels (17) (21.3 mg/L; standard error 7) and greater handgrip strength (3.12; 0.98) than patients receiving monotherapy (6.7 mg/L; 4.5 and 1.16; 0.3), respectively. Both groups were provided with a food supplement equivalent to 20% of the basal metabolic rate (BMR) in addition to dietary advice aimed at maintaining caloric intake equivalent to BMR.
Finally, a Swedish center with a history of using combination therapy for cancer cachexia compared standard care (SC) for cachexia in their center to SC plus low dose insulin in 138 patients with advanced GI malignancies (18). SC, included indomethacin for patients with elevated CRP, plus erythropoietin for anemia, and enteral or parenteral nutrition for decreased food intake (<90% and <80% respectively). Insulin treatment significantly improved survival (median 181 versus 128 days), stimulated carbohydrate intake as measured by 4-day food records, and increased body fat as measured by DEXA, particularly in trunk and leg compartments. There were no significant changes in muscle mass, tumor markers, REE, or spontaneous physical activity. Of note, SC also included beta-blockers for some patients possibly identified as having elevated REE.
Omega-3 supplements
Several systematic reviews have concluded that there is insufficient evidence for omega-3 supplements or fish oil providing benefit in cancer cachexia (19,20). In addition, a large randomized controlled trial (RCT) of more than 400 patients comparing MA to omega-3 supplements or the combination found no advantage to the addition of both agents. Omega-3 supplements, either alone or in combination with MA, did not improve weight or appetite better than MA alone (21). Survival and global QoL were not significantly different among treatment arms. With the exception of increased impotence in MA-treated patients, toxicity was comparable.
Omega-3 supplements were included in a large phase III RCT in 332 patients, showing combination therapy in cancer cachexia was more effective than any one individual component. Patients were randomized to 1 of 5 treatment arms: medroxyprogesterone or MA; oral supplementation with eicosapentaenoic acid (EPA); L-carnitine; thalidomide; and a combination of all the selected agents (22). The combination of all selected agents was the most effective treatment for LBM, REE, fatigue, appetite, IL-6, Glasgow Prognostic Score, and performance status (20).
A small trial combining melatonin and fish oil in patients with GI tumors produced stabilization of weight loss compared to either agent alone. The combination therapy consisted of 30mL of fish oil, providing 4.9 g of EPA and 3.2 g of docosahexanoic acid (DHA), plus 18 mg/day of melatonin. Although a synergistic effect was postulated, there was no change in the level of serum cytokines with the combination treatment (23).
Despite the systematic reviews, there is continued interest in the use of omega-3 enriched oral nutritional supplements (ONS). Preliminary trials have reported improved clinical outcomes including increased muscle mass, weight, and serum albumin (24,25). An PA enriched oral nutritional supplement compared to an isocaloric diet in NSCLC patients significantly improved protein intake, body composition, appetite, and decreased fatigue (26).
Other combinations
Other combinations shown to be more effective than monotherapy in randomized trials includes MA and thalidomide compared with MA alone. Combination therapy increased body weight by 2.27±6.62 kg compared to 1.19±2.57 kg in the control group. Fatigue also improved (2.57±4.67; –0.23±5.39), as did the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30EORTC (–7.93±13.20; –1.12±2.35), and the secondary endpoints of grip strength, Glasgow Prognostic Score, and pro-inflammatory cytokines (27). Toxicity was reported to be “relatively negligible” in both groups, possibly because of the low doses used (320 mg/day of MA and 100 mg/day thalidomide); however, other trials have reported intolerable side effects at higher doses of thalidomide (200 mg) (28). In theory, co-administration of an anabolic hormone such as testosterone with MA could improve appetite and counter some of MA’santi-anabolic and hypogonadal side-effects in males. The combination of MA and thalidomide has also been used in cachexia associated with chronic obstructive pulmonary disease (COPD) and HIV with inconsistent or mixed effects (29,30).
Non-pharmacologic interventions
Several trials have used a combination of nutritional counseling, ONS, and physical activity in combination with medications for cancer cachexia. The MENAC study is, however, the first to provide a standardized approach that includes dietary supplements, dietary counseling, and specific resistance and aerobic exercises in combination with a NSAID and omega-3 supplement. A multimodal approach requires an interdisciplinary team, and although specialist interdisciplinary teams are ideal and may include a physical therapist, dietician, and psychologist, there is evidence that non- specialist team members can also be effective in achieving improved clinical outcomes. For example, a nurse-led, simple “Walk-and-Eat” intervention (31) in a group of patients receiving outpatient chemoradiotherapy consisting of 20 minutes ambulation 3×/week and weekly nutritional advice resulted in significantly better handgrip strength, walk distance, and weight compared to a control group of usual care.
NIS
The role of symptom management and supportive care is fundamental in addressing the many contributors to decreased oral intake. They include early satiety, taste and smell alterations, mucositis, nausea, constipation, pain, dysphagia, fatigue, and depression (32). These symptoms are common (33), decrease energy intake (34), and respond to readily available, inexpensive medications, increasing oral intake and weight gain in up to one third of patients (32). Other metabolic abnormalities, such as hypogonadism, vitamin B12 deficiency, hypothyroidism, and hypoadrenalism, may also contribute to anorexia and muscle wasting in patients; however, low testosterone in males and vitamin D deficiency (35) appear to be most common. The importance of NIS was underscored by a study evaluating the impact of 17 symptoms on clinical outcomes in patients with head and neck cancer. Aggregate burden of symptoms was a significant independent predictor of reduced intake, weight loss, and survival (36). Importantly, these NIS may affect oral intake throughout the disease trajectory, occurring as a result of the symptoms and complications of advanced cancer, anticancer treatment, or even medical comorbidities.
Psychosocial interventions
Psychosocial support as part of a multimodal treatment has the potential to relieve distress and family conflict (37), support self-efficacy, decrease social isolation, and improve body image and adherence to treatment. A small study in the United Kingdom has demonstrated the feasibility of a nurse-delivered psychosocial intervention to mitigate weight- and eating-related distress in patients with advanced cancer and their careers. Components of the intervention included breaking the weight loss taboo, healing stories, managing conflict, advice on eating well, and supporting self-management. Distress among family members has also been reported to be increased. A multi-center Japanese study of 702 bereaved family members found high levels of eating-related distress and a need for education and support. About half of family members were distressed by the patient’s disappointment at their inability to eat, and 1 in 10 felt it was “useless” to consult medical staff about a daily diet (38-40). Psychological intervention and teaching cognitive reframing strategies also encourages patients to take control over eating habits, empowering the patient. A shift to conscious control over eating may be useful by reframing eating as a necessity (rather than a pleasure) for promoting positive outcomes such as slowing disease progression, tolerating side-effects of chemotherapy, and maintaining strength and stamina (41).
Nutritional supplements and dietary counseling
A multimodal approach emphasizing the need for increased caloric and protein intake is supported by trials that have included ONS as a component of the intervention and studies reporting that the majority of patients with advanced cancer and weight loss are consuming diets insufficient to maintain weight even in healthy individuals. A systematic review found older patients may be particularly susceptible, with up to 90% experiencing weight loss during chemotherapy. Dietary counseling, which includes increasing energy dense foods, meal frequency and the use of oral liquid nutritional supplements, can improve energy intake and body weight. However, there are few published studies, and a systematic review concluded there was not enough proof of benefit in patients identified as having cancer cachexia (42-44). However, a systematic review (45) and a meta-analysis (46) of nutrition interventions in oncology patients found counseling with/without ONS was associated with improvements in weight, BMI, energy intake and PG-SGA score.
Exercise
Exercise has the potential to improve muscle mass and strength, physical function (47), fatigue and QoL in patients with cancer cachexia. Exercise may exert its effects via modulation of muscle metabolism, insulin sensitivity, mitigation of tumor growth and decreasing levels of inflammation (48). However, despite strong justification for the use of resistance training and aerobic exercise (49), a systematic review found insufficient evidence to determine safety and effectiveness in patients with cancer cachexia (50). Exercise has been combined successfully with other modalities in small trials. Resistance exercise in combination with testosterone has a greater anabolic action than either intervention alone in age-related cachexia and has demonstrated similar benefits in HIV and COPD (51). In HIV-associated wasting, the combination of resistance training and testosterone in eugonadal men increases muscle mass (52). Exercise promises to be an important component of multimodal therapy by modulating expression of cytokines and perhaps acting in concert with anabolic hormones to improve strength, function, and QoL (53).
Current multimodal studies
The framework for a multimodality intervention outlined above has been incorporated into the design of two randomized trials. A phase II multimodal study of exercise, nutrition, and anti-inflammatory treatment for cachexia demonstrated feasibility and safety in patients receiving chemotherapy for incurable lung or pancreatic cancer. The follow-up phase III MENAC intervention is a multimodal, multi-site trial comprising ibuprofen (1,200 mg/day), omega-3 fatty acids (2 g EPA and 1 g DHA), ONS contributing 542 kcal and 30 g of protein, and a home-based exercise program consisting of resistance training three times/week in addition to aerobic training 2 times/week. Because patients in the early phase of cachexia are more likely to respond to therapy, the interventions will be initiated alongside chemotherapy and concomitant symptom management (54).
Conclusions
There will be ongoing debate regarding the specific therapies used in combination for cachexia until larger trials such as MENAC are able to confirm the efficacy of a particular multimodality intervention. The selection of individual agents may be based on clinical outcomes in single intervention trials or on studies using multimodal therapy. Despite the variations in composition, most multimodal regimens share a common purpose in simultaneously modulating the major mechanisms causing cachexia and including pharmacologic and non-pharmacologic interventions. Implementing a multimodal strategy successfully—especially one that includes exercise—requires early identification of at-risk patients. Modulating the aberrant inflammatory response, restoring endocrine homeostasis, and providing supportive care early in the disease trajectory offers the best prospect for improving weight, LBM, and physical function. A greater awareness of the CAS by clinicians and the development of biomarkers will facilitate timely diagnosis and initiation of therapy. In addition, multimodality therapies for cachexia need to be relatively safe—without a negative impact on survival or QoL—and must be accompanied by supportive care, which may include a team providing nutritional counseling, an exercise program, and optimal symptom management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer 2008;44:1124-32. [Crossref] [PubMed]
- Solheim TS, Laird BJA, Balstad TR, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care 2018;8:258-65. [Crossref] [PubMed]
- Naito T, Okayama T, Aoyama T, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer 2017;17:800. [Crossref] [PubMed]
- Wheelwright SJ, Darlington AS, Hopkinson JB, et al. A systematic review to establish health-related quality-of-life domains for intervention targets in cancer cachexia. BMJ Support Palliat Care 2016;6:307-14. [Crossref] [PubMed]
- Wheelwright SJ, Hopkinson JB, Darlington AS, et al. Development of the EORTC QLQ-CAX24, A Questionnaire for Cancer Patients With Cachexia. J Pain Symptom Manage 2017;53:232-42. [Crossref] [PubMed]
- Ribaudo JM, Cella D, Hahn EA, et al. Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 2000;9:1137-46. [Crossref] [PubMed]
- Blauwhoff-Buskermolen S, Ruijgrok C, Ostelo RW, et al. The assessment of anorexia in patients with cancer: cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer 2016;24:661-6. [Crossref] [PubMed]
- Fortunati N, Manti R, Birocco N, et al. Pro-inflammatory cytokines and oxidative stress/antioxidant parameters characterize the bio-humoral profile of early cachexia in lung cancer patients. Oncol Rep 2007;18:1521-7. [PubMed]
- Sakellariou GK, Lightfoot AP, Earl KE, et al. Redox homeostasis and age-related deficits in neuromuscular integrity and function. J Cachexia Sarcopenia Muscle 2017;8:881-906. [Crossref] [PubMed]
- Tan BH, Deans DA, Skipworth RJ, et al. Biomarkers for cancer cachexia: is there also a genetic component to cachexia? Support Care Cancer 2008;16:229-34. [Crossref] [PubMed]
- Johns N, Stretch C, Tan BH, et al. New genetic signatures associated with cancer cachexia as defined by low skeletal muscle index and weight loss. J Cachexia Sarcopenia Muscle 2017;8:122-30. [Crossref] [PubMed]
- Dev R, Del Fabbro E, Bruera E. Association between megestrol acetate treatment and symptomatic adrenal insufficiency with hypogonadism in male patients with cancer. Cancer 2007;110:1173-7. [Crossref] [PubMed]
- Pantziarka P, Bouche G, Sukhatme V, et al. Repurposing Drugs in Oncology (ReDO)-Propranolol as an anti-cancer agent. Ecancermedicalscience 2016;10:680. [PubMed]
- Solheim TS, Fearon KC, Blum D, et al. Non-steroidal anti-inflammatory treatment in cancer cachexia: a systematic literature review. Acta Oncol 2013;52:6-17. [Crossref] [PubMed]
- McMillan DC, Wigmore SJ, Fearon KC, et al. A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer 1999;79:495-500. [Crossref] [PubMed]
- Macciò A, Madeddu C, Gramignano G, et al. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol 2012;124:417-25. [Crossref] [PubMed]
- Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer 2007;59:14-20. [Crossref] [PubMed]
- Lundholm K, Körner U, Gunnebo L, et al. Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res 2007;13:2699-706. [Crossref] [PubMed]
- Ries A, Trottenberg P, Elsner F, et al. A systematic review on the role of fish oil for the treatment of cachexia in advanced cancer: an EPCRC cachexia guidelines project. Palliat Med 2012;26:294-304. [Crossref] [PubMed]
- Dewey A, Baughan C, Dean T, et al. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev 2007.CD004597. [PubMed]
- Jatoi A, Rowland K, Loprinzi CL, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 2004;22:2469-76. [Crossref] [PubMed]
- Mantovani G, Macciò A, Madeddu C, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010;15:200-11. [Crossref] [PubMed]
- Persson C, Glimelius B, Rönnelid J, et al. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: a randomized pilot study. Nutrition 2005;21:170-8. [Crossref] [PubMed]
- Abe K, Uwagawa T, Haruki K, et al. Effects of ω-3 Fatty Acid Supplementation in Patients with Bile Duct or Pancreatic Cancer Undergoing Chemotherapy. Anticancer Res 2018;38:2369-75. [PubMed]
- Yeh KY, Wang HM, Chang JW, et al. Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:41-8. [Crossref] [PubMed]
- Sánchez-Lara K, Turcott JG, Juárez-Hernández E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 2014;33:1017-23. [Crossref] [PubMed]
- Wen HS, Li X, Cao YZ, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy 2012;58:461-7. [Crossref] [PubMed]
- Wilkes EA, Selby AL, Cole AT, et al. Poor tolerability of thalidomide in end-stage oesophageal cancer. Eur J Cancer Care (Engl) 2011;20:593-600. [Crossref] [PubMed]
- Mulligan K, Zackin R, Von Roenn JH, et al. Testosterone supplementation of megestrol therapy does not enhance lean tissue accrual in men with human immunodeficiency virus-associated weight loss: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Endocrinol Metab 2007;92:563-70. [Crossref] [PubMed]
- Casaburi R, Nakata J, Bistrong L, et al. Effect of Megestrol Acetate and Testosterone on Body Composition and Hormonal Responses in COPD Cachexia. Chronic Obstr Pulm Dis 2015;3:389-97. [Crossref] [PubMed]
- Xu YJ, Cheng JC, Lee JM, et al. A Walk-and-Eat Intervention Improves Outcomes for Patients With Esophageal Cancer Undergoing Neoadjuvant Chemoradiotherapy. Oncologist 2015;20:1216-22. [Crossref] [PubMed]
- Del Fabbro E, Hui D, Dalal S, et al. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J Palliat Med 2011;14:1004-8. Erratum in: J Palliat Med 2011;14:1361. Noorhuddin, Zohra [corrected to Nooruddin, Zohra I]. [Crossref] [PubMed]
- Omlin A, Blum D, Wierecky J, et al. Nutrition impact symptoms in advanced cancer patients: frequency and specific interventions, a case-control study. J Cachexia Sarcopenia Muscle 2013;4:55-61. [Crossref] [PubMed]
- Bye A, Jordhøy MS, Skjegstad G, et al. Symptoms in advanced pancreatic cancer are of importance for energy intake. Support Care Cancer 2013;21:219-27. [Crossref] [PubMed]
- Dev R, Del Fabbro E, Schwartz GG, et al. Preliminary report: vitamin D deficiency in advanced cancer patients with symptoms of fatigue or anorexia. Oncologist 2011;16:1637-41. [Crossref] [PubMed]
- Farhangfar A, Makarewicz M, Ghosh S, et al. Nutrition impact symptoms in a population cohort of head and neck cancer patients: multivariate regression analysis of symptoms on oral intake, weight loss and survival. Oral Oncol 2014;50:877-83. [Crossref] [PubMed]
- Hopkinson JB. The Nourishing Role: Exploratory Qualitative Research Revealing Unmet Support Needs in Family Carers of Patients With Advanced Cancer and Eating Problems. Cancer Nurs 2018;41:131-8. [Crossref] [PubMed]
- McClement SE, Degner LF, Harlos M. Family responses to declining intake and weight loss in a terminally ill relative. Part 1: fighting back. J Palliat Care 2004;20:93-100. [PubMed]
- Hopkinson JB, Fenlon DR, Okamoto I, et al. The deliverability, acceptability, and perceived effect of the Macmillan approach to weight loss and eating difficulties: a phase II, cluster-randomized, exploratory trial of a psychosocial intervention for weight- and eating-related distress in people with advanced cancer. J Pain Symptom Manage 2010;40:684-95. [Crossref] [PubMed]
- Amano K, Maeda I, Morita T, et al. Eating-related distress and need for nutritional support of families of advanced cancer patients: a nationwide survey of bereaved family members. J Cachexia Sarcopenia Muscle 2016;7:527-34. [Crossref] [PubMed]
- Shragge JE, Wismer WV, Olson KL, et al. Shifting to conscious control: psychosocial and dietary management of anorexia by patients with advanced cancer. Palliat Med 2007;21:227-33. [Crossref] [PubMed]
- Nasrah R, Kanbalian M, Van Der Borch C, et al. Defining the role of dietary intake in determining weight change in patients with cancer cachexia. Clin Nutr 2018;37:235-41. [Crossref] [PubMed]
- Caillet P, Liuu E, Raynaud Simon A, et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: A systematic review. Clin Nutr 2017;36:1473-82. [Crossref] [PubMed]
- Balstad TR, Solheim TS, Strasser F, et al. Dietary treatment of weight loss in patients with advanced cancer and cachexia: a systematic literature review. Crit Rev Oncol Hematol 2014;91:210-21. Erratum in: Crit Rev Oncol Hematol 2015;94:146-8. [Crossref] [PubMed]
- Lee JL, Leong LP, Lim SL. Nutrition intervention approaches to reduce malnutrition in oncology patients: a systematic review. Support Care Cancer 2016;24:469-80. [Crossref] [PubMed]
- Baldwin C, Spiro A, Ahern R, et al. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:371-85. [Crossref] [PubMed]
- Oldervoll LM, Loge JH, Lydersen S, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist 2011;16:1649-57. [Crossref] [PubMed]
- Moreira VM, da Silva Franco CC, Prates KV, et al. Aerobic Exercise Training Attenuates Tumor Growth and Reduces Insulin Secretion in Walker 256 Tumor-Bearing Rats. Front Physiol 2018;9:465. [Crossref] [PubMed]
- Alves CR, da Cunha TF, da Paixão NA, et al. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci 2015;125:9-14. [Crossref] [PubMed]
- Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: Executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle 2015;6:208-11. [Crossref] [PubMed]
- Lambert CP, Sullivan DH, Freeling SA, et al. Effects of testosterone replacement and/or resistance exercise on the composition of megestrol acetate stimulated weight gain in elderly men: a randomized controlled trial. J Clin Endocrinol Metab 2002;87:2100-6. [Crossref] [PubMed]
- Grinspoon S, Corcoran C, Parlman K, et al. Effects of testosterone and progressive resistance training in eugonadal men with AIDS wasting. A randomized, controlled trial. Ann Intern Med 2000;133:348-55. [Crossref] [PubMed]
- Lewis MI, Fournier M, Storer TW, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol 2007;103:1299-310. [Crossref] [PubMed]
- Solheim TS, Laird BJA, Balstad TR, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:778-88. [Crossref] [PubMed]


