What are the main palliative care symptoms and concerns of older people with multimorbidity?—a comparative cross-sectional study using routinely collected Phase of Illness, Australia-modified Karnofsky Performance Status and Integrated Palliative Care Outcome Scale data
Introduction
Estimates suggest that worldwide 19 million people need palliative care, 66% of whom have non-cancer conditions (1). Current trends suggest that older people with progressive long-term conditions will be increasingly prevalent amongst those in need of palliative care (2). These patients may have needs different from those of younger patients with malignancy who are those more typically seen by specialist palliative care services (3,4). Whilst there has been a shift to offering palliative care to other single non-cancer diseases (5,6), this does not routinely extend to those with multimorbidity, typically those dying in old age (7). Worldwide, multi-morbidity is increasing dramatically which requires a new and different clinical and service response (8). Due to the interplay of multimorbid long-term conditions and frailty, older people have very different patterns of illness (9,10). Their periods of illness are often protracted, with episodic crisis events, any one of which may be the terminal event. Older people with advanced illness have preferences for care which are strongly influenced by family and care context (11), and will likely require different configurations of palliative care services (12-14). The World Health Organization (1) calls for a strengthening of palliative care as a component of care across long-term conditions and older age groups. We need therefore better to understand the palliative care needs of older people with multimorbidity and adapt or design services accordingly. Currently, evidence is limited and poor (15), so the data captured by innovative services such as the one described here are both important and timely.
To describe the clinical characteristics, symptoms and other concerns of older people with multi-morbidity referred to a new palliative care service; by comparing this service population with people receiving standard community-based specialist palliative care.
Methods
Design
Comparative cross-sectional study, using routinely collected demographic, clinical, and point-of-care patient-level outcomes data, with illustrative composite case studies (16).
Setting and service context
St Christopher’s Hospice, London UK, serves a diverse population of 1.5 million people, delivering care largely in the community, and predominately via multi-disciplinary, nurse-led, specialist palliative home care teams. Within the London borough of Bromley, St Christopher’s has developed an innovative home care service, Bromley Care Co-ordination (BCC), primarily for older people with palliative care needs who do not meet the criteria for referral to specialist palliative care services (17). BCC, commissioned by a group of community doctors in 2014, provides timely and coordinated end-of-life care to patients with progressive illness and/or frailty, thought to be in the last year of their life. See Supplementary 1 for further information regarding the BCC service and Supplementary 2 for referral criteria for both BCC and the standard community-based palliative care services-referred to as standard care, SC.
The Outcome Assessment and Complexity Collaborative (OACC)
Within both the new BCC service and the standard community-based specialist palliative care team patient outcome data is collected using the Outcome Assessment and Complexity Collaborative (OACC) (18,19) suite of outcome measures (see Supplementary 3). This research collaborative, led by the Cicely Saunders Institute at King’s College London, seeks to measure and improve care by implementing patient-level, point of care outcome measures into routine specialist palliative care . OACC measures used in this analysis are:
- Palliative Care Phase of Illness (POI) (20): POI is a clinician-completed measure that describes four distinct clinical stages of a palliative patient’s illness: Stable, Unstable, Deteriorating, and Dying (or Deceased). Patients are classified according to their care needs and the needs of their family, and the suitability of the current palliative care plan. The phases are non-hierarchical, with patients moving between Phases in any direction or sequence, according to need.
- Australia-modified Karnofsky Performance Status (AKPS) (21). AKPS is a clinician-completed measure of basic functional status. The patient is assessed on three dimensions: activity, work, and self-care and given an overall performance score in intervals of 10 from 100 fully functioning to 0 dead.
- Integrated Palliative Care Outcome Scale (IPOS) (22,23) IPOS is a measure of symptoms, problems and concerns known to affect patients with advanced illness. The questionnaire includes free text responses and 17 items on a Likert scale, from 0 absent to 4 overwhelming. ‘There are two IPOS versions: a patient version, completed by patients themselves (with help if needed, from family or staff) and a staff version (completed by professionals). IPOS is a valid and reliable measure (24) directly underpinned by those symptoms and concerns most often reported by people with advanced illness.
Data collection
An ‘episode of care’ is defined as beginning with the first contact a patient has with a service after referral and ends when the patient moves setting (e.g., admission to a hospice bed or hospital), is discharged, or dies. Discharge includes patients transferred to another service within St Christopher’s Hospice. Data was collected on complete episodes of care beginning between January 2016 and August 2017. Episodes that began before January 2016 were excluded. Data up to November 2017 were used to allow a 3-month follow-up period to capture end of episode; episodes that ended after this period are excluded from analysis of length of episode. Phase of illness is assessed along with AKPS and IPOS at the start of every episode of care. Subsequently, Phase of illness is assessed during every contact with the patient; when Phase changes, a further collection of AKPS and IPOS is triggered. Additional collections of IPOS and AKPS may be made between Phase changes as part of routine clinical care. Patients may move between settings, sometimes several times, individual patients may have multiple episodes of care and within each episode, multiple Phases of Illness may be recorded.
Data analysis
Patients seen by BCC and Standard Care were described in terms of demographics and primary diagnosis. Whilst secondary diagnoses are vital, the current quality of collected data is too poor to be included in analysis. Episodes of care were described in terms of length, status (alive or died) and destination at the end of episode, and the number, distribution and length of first Phase of Illness, and AKPS at first contact. Average (mean and SD) scores for patients with complete data (listwise deletion) for all 17 IPOS items was compared for the two services. The results were tested in a sensitivity analysis using pairwise deletion. Chi-square test for categorical variables, and Mann-Whitney or 2-sample t-test for numerical variables were used to test for differences between the groups. A Bonferroni correction was applied to account for multiple testing, generating a new significance level of P<0.002 (0.05/33). Patients could be seen by both services, however overlap was expected to be minimal and risk of type 2 errors was small, therefore no adjustment was deemed necessary (25). Missing IPOS data at Phase change was imputed on an item by item basis from an IPOS completed within 3 days of Phase change. All analysis was carried out in Stata v13 (26).
Case studies
Composite case studies (16) of patients with multiple morbidities derived from reviewing BCC patient notes and discussion with the multi-disciplinary BCC team, provide further in-depth illustration of patient characteristics and clinical needs.
Ethics
Analysis was based on fully pseudonymised patient records and is therefore exempt from ethical approval according to the Information Commissioner’s Office guidance and those of the King’s College London Research Ethics Committee. Caldicott Guardian approval was received prior to the evaluation for the analysis of pseudonymised clinical data to evaluate this service. St Christopher’s Hospice ethical committee approved composite case study construction.
Results
Between January 2016 and August 2017, 815 patients began an episode of care with the BCC service and 1,254 with Standard Care; 225 (12%) patients were seen by both services.
Table 1 presents the characteristics of the patients seen. Comparing across the two services, a larger proportion of BCC were female, 49.5% and 63.4% respectively. BCC patients were on average older (median age 88) than those who received Standard Care (median age 78). 71.6% of the patients seen by Standard Care had a cancer diagnosis, compared to 16.5% of patients seen by BCC. A larger proportion of BCC patients had dementia (20.5% in BCC versus 4.5% in SC), heart failure (14.1% in BCC versus 3.7% in SC), and other non-cancer conditions (34.9% in BCC versus 14.0% in SC). Within both patient groups, ethnicity was predominantly White British.

Full table
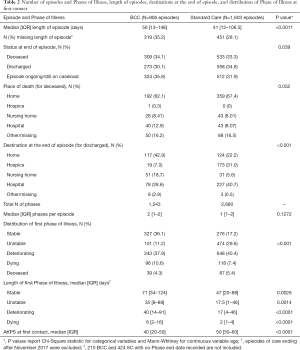
906 episodes of care were recorded for BCC and 1,603 for Standard Care; Table 2 describes these episodes of care. Episodes of care were on average longer in the BCC service, median 56 days, compared to median 41 days in Standard Care (P<0.0011). For episodes of care ending in discharge, a larger proportion of patients seen by BCC were discharged home (42.9% in BCC versus 22.2% in SC), and a smaller proportion were discharged into hospital (40.7% and 28.6% respectively) and into hospice (31.0% and 7.0% respectively) (P<0.001). More patients seen by BCC were stable at the start of their episode of care, 36.1% compared to 17.2% in Standard Care (P<0.001). Length of deteriorating Phase appears to be longer in BCC episodes, median 40 days compared to a median of 17 days in Standard Care (P<0.0001). The functional status of BCC patients at the start of their episode of care was lower, median AKPS 40, compared to median AKPS 50 in Standard Care (P<0.0001). Composite BCC Case study 1 illustrates a “typical” presentation of a BCC patient at first assessment.
Full table
Composite case study 1: “Typical” BCC patient at first assessment
Figure 1 depicts the trajectory of Phase changes across the two services from the first to the second Phase; not all patients have a second Phase of illness recorded and not all episodes that end in death have a deceased Phase then recorded. Across both services the trajectory of Phase changes may occur in any order, for example some patients in the dying Phase at Phase 1 change to a stable, unstable or deteriorating Phase at Phase 2. For patients who were unstable at Phase 1, 24% of BCC patients and 12% of Standard Care patients became stable at Phase 2.
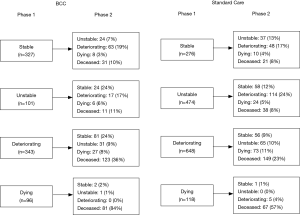
There appear to be a wider variation in the direction of POI across the two phases in BCC compared to Standard Care e.g., phase change from deteriorating to stable in BCC is 24% and 9% in SC. Composite BCC case study 2 illustrates the wide variation and direction of Phases of Illness of some BCC patients from referral to death. It is often the accumulation of changes across physical, cognitive and social domains that drive the phase change in BCC patients.
Composite BCC case study 2: a “typical” trajectory of Phase change for BCC patients
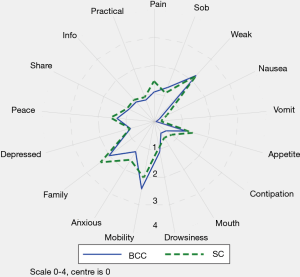
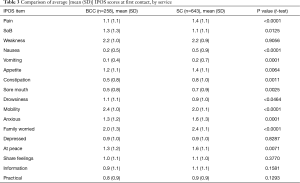
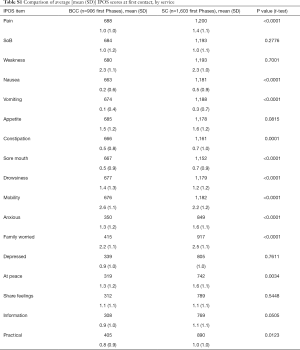
Table 3 and Figure 2 describe the average symptom profile at first contact within an episode of care for each service; for BCC and Standard Care respectively, complete IPOS scores were recorded for 258 and 643 first Phases. Patients across the two services had a similar symptom profile at first contact. However, compared to BCC patients, Standard Care patients had on average slightly more pain, nausea, vomiting, constipation, anxiety and family concern, and slightly less mobility concerns. Results were similar in the pairwise complete case analysis (see Supplementary 4).
Full table
Discussion
This report illustrates that older people with multimorbidity, approaching the end of life, have considerable palliative care needs. Symptom burden and concerns on first contact were surprisingly similar between BCC, our new service—set up primarily for older people, who do not meet the criteria for referral to specialist palliative care and those people receiving Standard Care. Using IPOS, only seven items have a statistically significant difference across the BCC and Standard Care groups. Pain, nausea, vomiting and sore mouth, family worry and anxiety are more frequently rated for Standard Care patients and mobility was more frequently rated for BCC patients. The expectations were for differences that are more widespread across the two groups, since IPOS was developed around the main palliative care concerns of people (including older people) with advanced single diseases, e.g., Cancer (27), Renal disease (28), Chronic Obstructive Airways Disease (29) and Heart Failure (30).
Evidence is emerging that older people with multimorbidity may experience pain and emotional distress similar to that of patients with cancer at the end of life (31). However, data on the specific palliative care needs of people with multimorbidity is minimal; in part due to the history of palliative care in cancer, in part because multimorbidity is under-evidenced in all clinical areas and in part because definitional controversies hamper identification of people with multimorbidity (8) and frailty (32). As clinicians, we often experience BCC patients as having more symptoms of “deficit” e.g., lack of energy, poor functional status, whilst the traditional specialist palliative care patients have more acute and intense symptoms e.g., pain. There are almost certainly other aspects of care needs, potentially specific to older people with multimorbidities, which are not identified through POI, AKPS and IPOS. More work is needed to understand whether there are specific palliative care symptom clusters, which are commonly associated with older people with multi-morbidity, to determine our response to need. For example, the cluster of increasing weakness, poor mobility and poor appetite has a similar symptom profile to Fried et al.’s (33) frailty phenotype model; i.e., unintentional weight loss, exhaustion, muscle weakness, slowness while walking, and low levels of activity. Functional interventions are a key mediator of frailty (34). Specialist palliative care can sometimes focus’ on disease and not disability, assuming functional decline to be an inevitability of advanced disease (35). Our results confirm the importance of rehabilitative palliative care (36).
We need to understand more about the evolution of needs and symptoms over time for BCC patients and the relationship with multimorbidity. Whilst the focus in this paper is across only two phases (as measured by Phase of Illness), it does appear that BCC patients are more likely to improve back to stability after a crisis (from unstable or deteriorating) than Standard Care patients. This may indicate their uncertain illness trajectory (37) sometimes referred to as ‘progressive dwindling’ (9). Patients with severe frailty typically have a low functional level which remains as a steady state, with only a slight deterioration as death approaches (38). BCC patients in this study were more affected by poor mobility as measured on IPOS and had lower AKPS scores on entering the service (see Table 1). Thus, change in AKPS as an indicator of decline may be less meaningful in older people with frailty and or multimorbidities. Clinically, the BCC population seems to have a different momentum and pace of change, clinicians’ report that at times it can be harder to register change and the effect of specialist palliative care interventions. This may beg the question of how we might develop different services capable of responding to subtle changes in need independent of the traditional diagnostic pointers.
Destinations at end of phase for people in BCC are more community focussed- home and care home, as opposed to Standard Care patients. This may be a matter of preference or imply that primary diagnosis on presentation may dictate a different service response e.g., people with malignancy may have stronger pre-existing relationships with acute care; or people with long-standing functional dependence may require institutional support. Projections of place of death in the UK based on current trends predict number of deaths in care homes and homes will increase by 108.1% and 88.6%, with care home the most common place of death by 2040 (39). However specialist palliative care home support in the UK is inconsistent (40).
We do not yet know whether the similarities and differences in the symptom profile across the two cohorts will continue throughout the illness trajectory. Larger cohorts and longer observation times will increase our understanding and provide the strongest case for routine outcome measurement and its refinement as part of clinical practice. Patient-reported palliative care outcome measurements (PROMs) are considered the best way both to engage patients actively in assessing the care they receive and to evaluate the effectiveness of palliative care services in meeting that need (41). However, we need more and higher quality evidence from PROMS in non-cancer populations, across a wider range of settings (42). Long-term care facilities are developing such evidence, e.g., Pan European Progress Quality indicators and associated outcomes, including end of life (43) and IPOS-Dem for caregivers of people with dementia in long term care (44), but further testing and extension into wider community settings is needed.
The results of this paper suggest that it may be different patterns of symptoms, rather than overall level of need itself, which is different between older people with multimorbidity and standard specialist palliative care patients. The necessity now is to advance this evidence and develop services responsive to the differing presentations of need.
Study limitations
The clinical data used in this study is limited by missing data. In particular, large proportions of missing items were found for IPOS (72% of BCC and 60% of SC had incomplete IPOS data at first Phase). Our analysis of IPOS was restricted to patients with complete data across all IPOS items, and we do not know if those with complete IPOS scores are different from those without.
The pattern of missing data across the IPOS items (see Table S1), with more missing data present for the psycho-social items, suggest that staff may find completion of these items more challenging than the physical items for which there is less missing data.
Length of Phase can be artificially lengthened if a Phase change goes undetected. This might occur either due to staff being unsure about the application of the Phase measure or when Phase changes are missing during gaps between clinical contact. Within this single dataset, it is not possible to evaluate how many Phase changes have been missed. Further evaluation of the application of Phase across services in St Christopher’s Hospice is needed to support consistent use of the measure.
Despite the limitations of this clinical data, this is the first study known to us to use patient centred outcomes data to describe the population of older people with multimorbidity. The major strength of this study is to shed light on the variation in need between this growing population and the patients more typically seen in specialist palliative care.
Conclusions
This study characterizes BCC patients on entry to this new palliative care service innovation. They are older, mostly dying with non-malignancy, yet with a similar overall symptom burden compared to those seen in a more standard specialist palliative care service. Currently, the population of older people with multimorbidity is not routinely recognized as having specialist palliative care needs. However, it would seem that whilst needs of referral might be similar, presentation and patterns of symptoms may differ over time. Longitudinal prospective data are needed to understand BCC patient routes through the service compared to Standard Care, changes in type and severity of symptom burden and concerns and the relationship with frailty.
Supplementary 1
Bromley Care Coordination (BCC)
The service aims to address the inequalities of access to services for dying patients to prevent unnecessary hospital admissions, to help people die with dignity in their place of choice and to provide support for their families and carers). BCC is a nursing-led service, with the community doctor taking medical responsibility for the patient. The team consists of Clinical Nurse Specialists, Health Care Assistants, a line manager and administrators. Other hospice services are available as necessary to meet patient needs. However, interdependencies with non-specialist palliative care providers are core to the service e.g., community nurses, geriatricians, allied health professionals and social care providers. Those using the service can access advice and help around the clock, 365 days a year.
The service averages a caseload of 300 people at any one time, of whom 85% have a non- cancer diagnosis and 63% are aged over 85. To date, outcomes include reduction of deaths in hospital (76% of patients have died at home, compared with the average in the borough of 23%) and reduction in inappropriate hospital admissions (BCC 2016-2017 service data). It has also increased patient and family satisfaction and improved anticipatory care planning. Resource implications of the proposed model include an increase in key working some patients, rather than the original plan, solely to assess and refer on, to other services. This is in part due to the lack of services for some patient groups e.g., people with dementia and long-term neurological disorders who have high levels of dependency and uncertainty around deterioration. In part, the non-existent or fragile social networks of people living on their own or with an elderly spouse, makes the on-going connection with hospice care of extra importance.
Supplementary 2
Referral Criteria for BCC and Standard Care community-based palliative care services
Referral Criteria for BCC community-based palliative care service
Any person thought to be in the last year(s) of life.
Indications for referral include:
- People with an Electronic Frailty Index Score of >0.36—severe frailty, (over 13 deficits) Multiple admissions to hospital in the last year;
- Increasing uncertainty;
- Deterioration;
- Precarious social support/network/carer burden and escalation of concern;
- Would benefit from ACP or discussions about the future;
- Requires care co-ordination—currently falling between services;
- A combination of two or more long term comorbidities (rather than an acute event) including;
- Cancer
- Cardiac diseases (heart failure)
- Dementia
- Endocrine (e.g., diabetes)
- Neurological diseases (e.g., Parkinson’s, multiple sclerosis)
- Renal failure
- Respiratory disease
Referral Criteria for Standard Care community-based palliative care service*
Patients are admitted into the service with advanced cancer, motor neurone disease, HIV or any other advanced, progressive and life limiting non-malignant disease. The complexity of the illness needs the services of a specialist team to achieve control of symptoms and to offer social, psychological and spiritual support to the patient and family. All referrals are prioritized according to the complexity of problems presented.
*2014–currently under review
Supplementary 3
The Outcome Assessment and Complexity Collaborative (OACC), a research project led by the Cicely Saunders Institute KCL London, seeks to implement outcome measures into routine clinical care to measure, demonstrate and improve palliative care. OACC consists of a suite of fit-for-purpose measures including:
Phase of Illness (POI)—developed in Australia about 20 years ago and now commonly used in many countries. POI describes four distinct clinical stages of a palliative patient’s illness: stable, unstable, deteriorating, and dying (and deceased). Classified according to the care needs of the patient and the family, and the suitability of the current care plan. POI is validated for inter-rater reliability and acceptability amongst clinicians (45).
Australia-modified Karnofsky Performance Status (AKPS) (21). The patient’s overall performance status is assessed in three dimensions: activity, work and self-care scores in intervals of 10 from 100 (fully functioning to 0—dead). It provides basic functional status information and can be predictive of survival.
Integrated Palliative Care Outcome Scale (IPOS) (22) (Herne and Higginson 1999) is a measure of global symptom burden, which includes items that measure physical, psychosocial, social and spiritual domains in line with an impeccable holistic assessment. IPOS has undergone intensive testing in both cancer and non-cancer settings. IPOS has 17 items, common symptoms and problems in palliative population; measures recorded on a Likert scale, 0 absent to 4 overwhelming.
The timing of OACC measures has developed since its inception in 2013. Currently Phase of Illness, AKPS and IPOS are collected at first point of contact and then at a change of phase or illness or every three months- whichever is sooner. In order to demonstrate clinical difference, if any, over time, data is collected ideally at the beginning and end of every phase and at every phase change. Ideally, outcome measures are captured before the patient is discharged.
Supplementary 4: sensitivity analysis, pairwise complete case analysis
Full table
Acknowledgements
The OACC project is led by the Cicely Saunders Institute. It is funded by the Guy’s and St Thomas’ Charity and supported by project BuildCARE. OACC is working in collaboration with the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Palliative and End of Life Care Theme. The Collaboration for Leadership in Applied Health Research and Care (CLAHRC) South London is part of the National Institute for Health Research (NIHR), and is a partnership between King’s Health Partners, St. George’s, University London, and St George’s Healthcare NHS Trust. Hospice UK, who are working in partnership with the Cicely Saunders Institute to support the OACC project. Bromley Clinical Commissioning Group, St Christopher’s Hospice-BCC Clinical Team, Data analysts and all study participants.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Caldicott Guardian approval was received prior to the evaluation for the analysis of pseudonymised clinical data to evaluate this service. St Christopher’s Hospice ethical committee approved composite case study construction.
References
- WHA67.19. W, Strengthening of palliative care as a component of comprehensive care throughout the life course. Geneva: World Health Organization, 2014.
- Etkind SN, Bone AE, Gomes B, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017;15:102. [Crossref] [PubMed]
- Bausewein C, Booth S, Gysels M, et al. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med 2010;13:1109-18. [Crossref] [PubMed]
- Bennett MI, Ziegler L, Allsop M, et al. What determines duration of palliative care before death for patients with advanced disease? A retrospective cohort study of community and hospital palliative care provision in a large UK city. BMJ Open 2016;6. [Crossref] [PubMed]
- Murtagh FE, Addington‐Hall JM, Higginson IJ. End‐Stage Renal Disease: A New Trajectory of Functional Decline in the Last Year of Life. J Am Geriatr Soc 2011;59:304-8. [Crossref] [PubMed]
- Goodlin SJ, Hauptman PJ, Arnold R, et al. Consensus statement: palliative and supportive care in advanced heart failure. J Card Fail 2004;10:200-9. [Crossref] [PubMed]
- Rosenwax L, Spilsbury K, McNamara BA, et al. A retrospective population based cohort study of access to specialist palliative care in the last year of life: who is still missing out a decade on? BMC Palliat Care 2016;15:46. [Crossref] [PubMed]
- The Academy of Medical Sciences. Multimorbidity: a priority for global health research, 2018 April
- Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at the end of life. JAMA 2003;289:2387-92. [Crossref] [PubMed]
- Boyd K, Murray SA. Recognising and managing key transitions in end of life care. BMJ 2010;341:c4863. [Crossref] [PubMed]
- Etkind SN, Bone AE, Lovell N, et al. Influences on Care Preferences of Older People with Advanced Illness: A Systematic Review and Thematic Synthesis. J Am Geriatr Soc 2018;66:1031-9. [Crossref] [PubMed]
- Pollock K, Seymour J. Reappraising ‘the good death’for populations in the age of ageing. Age Ageing 2018;47:328-30. [Crossref] [PubMed]
- Hall S, Petkova H, Tsouros AD, et al. Palliative Care For Older People: Better Practices, 2011.
- Evans C, Ellis-Smith C, Nicholson C, et al. Rapid Scoping Review of Service Delivery Models to Maximise Quality of Life for Older People at the End of Life Prepared for the World Health Organisation (WHO) by King’s College London. 2018. Available online: http://www.who.int/kobe_centre/mediacentre/news/EOLC_report/en/
- Petrillo LA, Ritchie CS. The challenges of symptom management for patients with multimorbidity in research and practice: a thematic review. Prog Palliat Care 2016;24:262-7. [Crossref] [PubMed]
- Roberts NK, Williams RG, Klingensmith M, et al. The case of the entitled resident: A composite case study of a resident performance problem syndrome with interdisciplinary commentary. Med Teach 2012;34:1024-32. [Crossref] [PubMed]
- Nicholson C. Palliative Care, Frailty and Older People In: MacLeod RaLdB, L, editor. Textbook of Pallaitive Care in Press: Springer, 2018.
- Witt J, Murtagh FE, Daveson BA, et al. International advances in outcome measurement in palliative care: one step closer to cross-national comparisons of routinely collected outcome data in palliative care. Eur J Palliat Care 2015.
- . Available online: https://www.kcl.ac.uk/nursing/departments/cicelysaunders/research/studies/oacc/index.aspxOACC Project.
- Eagar K, Green J, Gordon R. An Australian casemix classification for palliative care: technical development and results. Palliat Med 2004;18:217-26. [Crossref] [PubMed]
- Abernethy AP, Shelby-James T, Fazekas BS, et al. The Australia-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice BMC Palliat Care 2005;4:7. [ISRCTN81117481]. [Crossref] [PubMed]
- Hearn J, Higginson I. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care 1999;8:219-27. [Crossref] [PubMed]
- Palliative care Outcome Scale website. Cicely Saunders Insititute. Available online: www.pos-pal.org
- Murtagh F, Ramsenthaler C, Firth A, et al. A Brief, Patient- and Proxy-reported Outcome Measure for the Adult Palliative Care Population: Validity and Reliability of the Integrated Palliative Outcome Scale (IPOS) (in EAPC 2016: Abstracts). Palliat Med 2016;30.
- Hayes LJ, Berry G. Comparing the part with the whole: should overlap be ignored in public health measures? J Public Health (Oxf) 2006;28:278-82. [Crossref] [PubMed]
- StataCorp. 2013. Stata Statistical Software: Release 13. College Station TSL.
- Osborne TR, Ramsenthaler C, Schey SA, et al. Improving the assessment of quality of life in the clinical care of myeloma patients: the development and validation of the Myeloma Patient Outcome Scale (MyPOS). BMC cancer 2015;15:280. [Crossref] [PubMed]
- Saini T, Murtagh FE, Dupont P, et al. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med 2006;20:631-6. [Crossref] [PubMed]
- Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014;2:979-87. [Crossref] [PubMed]
- Kane PM, Daveson BA, Ryan K, et al. Feasibility and acceptability of a patient-reported outcome intervention in chronic heart failure. BMJ Support Palliat Care 2017;7:470-9. [Crossref] [PubMed]
- Matthews FE, Spiers G, Hanratty B. Do people with frailty have palliative and end of life care needs? Society for Academic Primary Care. 47th Annual Scientific Meeting. London 10-12th July. Available online: https://sapc.ac.uk/conference/2018/abstract/do-people-frailty-have-palliative-and-end-of-life-care-needs_p1-17
- Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013;68:62-7. [Crossref] [PubMed]
- Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255-63. [Crossref] [PubMed]
- Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database System Rev Implement Rep 2018;16:140-232. [Crossref] [PubMed]
- Jerant AF, Azari RS, Nesbitt TS, et al. The TLC model of palliative care in the elderly: preliminary application in the assisted living setting. Ann Fam Med 2004;2:54-60. [Crossref] [PubMed]
- Leslie P, Sandsund C, Roe J. Researching the rehabilitation needs of patients with life-limiting disease: Challenges and opportunities. Prog Palliat Care 2014;22:313-8. [Crossref]
- Lloyd A, Kendall M, Cardiff E, et al. Why do older people get less palliative care than younger people? J Palliat Care 2016;23:132-7.
- Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc 2003;51:451-8. [Crossref] [PubMed]
- Bone AE, Gomes B, Etkind SN, et al. What is the impact of population ageing on the future provision of end-of-life care? Population-based projections of place of death. Palliat Med 2018;32:329-36. [Crossref] [PubMed]
- Dixon J, King D, Matosevic T, et al. Equity in the provision of palliative care in the UK: review of evidence. 2015.
- Bausewein C, Daveson B, Benalia H, et al. Outcome measurement in palliative care: the essentials. PRISMA 2011.
- Etkind SN, Daveson BA, Kwok W, et al. Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: does it make a difference? A systematic review. J Pain Symptom Manage 2015;49:611-24. [Crossref] [PubMed]
- European Centre for Social Welfare, Policy and Research Measuring Progress: Indicators for care homes. 2010.
- Ellis-Smith C, Evans CJ, Murtagh FE, et al. Development of a caregiver-reported measure to support systematic assessment of people with dementia in long-term care: The Integrated Palliative care Outcome Scale for Dementia. Palliat Med 2017;31:651-60. [Crossref] [PubMed]
- Masso M, Allingham SF, Banfield M, et al. Palliative care phase: inter-rater reliability and acceptability in a national study. Palliat Med 2015;29:22-30. [Crossref] [PubMed]