Economic outcomes in palliative and end-of-life care: current state of affairs
Brief summary of recent research
The evidence on economic outcomes and palliative care has been the subject of multiple literature reviews in the last 5 years. The definitive systematic review of costs and cost-effectiveness associated with all models of palliative care found 46 studies with a general pattern of cost-saving but heterogeneity of everything: different populations (e.g., by age, diagnosis, prognosis), different national health systems and societies, different interventions and settings, different approaches to cost perspectives, and different approaches to managing the statistical properties of utilization data (1).
A second review across all settings examined cohort decedent studies—those focused on patients known to have died, comparing the impact of treatment choices on outcomes in the last weeks and months of life. A remarkable 78 studies of cancer decedents were included (since the authors also report an increasing prevalence of such studies to 2011, the number now might be double that) (2). Again, palliative care was associated with lower utilization and costs, yet the authors noted that palliative/hospice services were not received by two-thirds of the patients in the studies.
In the hospital setting, a review focusing on palliative care consultation (PCC) teams encountered fewer heterogeneity problems with ten studies from the US evaluating the impact of a more-or-less-standardized intervention (3). However, differences in costing and statistical methods again prevented meta-analysis, particularly in the context of a recurring weakness to established methods (4), and so the key finding was again a general pattern of cost-saving. A review of palliative care and intensive care unit admissions for hospitalized adults also found an association with lower utilization (5).
With respect to homecare, a Cochrane review of palliative care services for adult patients and their family caregivers found only six cost-effectiveness studies and inconclusive evidence (6). And a review of financial implications for caregivers found that the magnitude of costs for family members is consistently large but there is limited evidence on treatment effects of palliative care to ameliorate these high costs (7).
Despite the substantive differences both within and between these reviews, four common themes are clear. First, there is an almost complete reliance on the ‘cost’ component of cost-effectiveness analysis. Studies typically examine intervention impact on costs, and assume that cost-savings are beneficial; the assumption is that outcomes are always at least as good for palliative care patients as usual care patients. Second, there is a narrow perspective on costs. Studies overwhelmingly examine routinely collected costs from either the payer’s or hospital’s perspective (but rarely examined side by side), and costs to patients and their families (informal costs) are largely under-examined. Third, there is a very limited time window of analyses. Evidence comes mostly from studies of hospital stays and/or healthcare utilization at the end of life, which are more representative of rapidly progressing diseases such as cancer, and less representative of the care of persons with increasing frailty over many months or years. Intertwined with these characteristics is the fact that this area of research is predominantly conducted in North American settings (some in Canada and the bulk in the US)—up to 100% of studies in the reviews described above. The cause of this is unclear, but the themes detected in these reviews may certainly be related to the national setting in which the clinical interventions and outcomes are defined and measured.
Widening our scope of enquiry
As our introduction notes, current evidence on the economic outcomes from palliative care has a consistent conclusion—interventions are generally cost-saving—but also consistent limitations around outcomes of interest, cost perspective and time window of analysis.
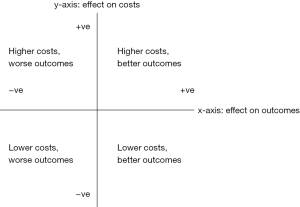
From first principles, economic evaluation is the examination of two different options’ effect on both costs and outcomes (8,9). A treatment’s effect against a comparator can be placed in Figure 1: if a strategy reduces costs and improves outcomes (south-east quadrant) then it is a dominant strategy; you would be foolish not to pursue it. Conversely if it increases costs and reduces outcomes (north-west) then it is inferior and should clearly be avoided. More difficult decisions are made in the north-east and south-west quadrants where an intervention improves outcomes at a higher cost, or saves money for inferior outcomes, and judgements are necessary. Are extra costs for improved outcomes worth paying for? Are worse outcomes tolerable given the savings?
Palliative care is most often defined as an additional service offered to patients in need, which does not require the cessation of disease-focused care (a major exception being hospice care in the US, where it is generally structured to be mutually exclusive with disease-focused care). Consistent with that definition, most clinical trials of palliative care use “usual care” as the control [35 of 43 trials in a recent systematic review (10)]—does the addition of this intervention improve patient outcomes, compared to the status quo? And (or) does it reduce the costs of care?
Reviews of trials of the impact of palliative care on clinical and patient-reported outcomes have generally found better outcomes, which would place palliative care in one of the two east-side quadrants in the figure. And as noted earlier, recent reviews found a consistent theme of cost-reduction when palliative care was involved, which would place it in the two south-side quadrants. However, few studies have measured these two kinds of outcomes together, and even where they have, have not usually found positive impacts in both domains in the same study.
The measurement of costs and patient reported outcomes together is critical for palliative care, because of the heterogeneity of interventions, settings, and patients that fall under the “palliative care” label. Are the cost-savings and positive patient outcomes produced from the same kinds of interventions, in the same settings, and among the same kinds of patients? Or is one kind of intervention producing cost-savings, and another kind (or different setting or population) producing positive patient outcomes?
If palliative care was as standardized as a simple pill that could be mass-produced and administered in the same fashion across multiple studies, then this would not be an issue. As a complex intervention in which context, timing and delivery are important factors, palliative care is anything but a simple pill (11).
A second critical limitation of economic analyses of palliative care to date, compared to guidelines for such studies, is the cost perspective used. The societal perspective to costs is widely accepted as the optimal approach (8,9) and suggests that costs be measured from all relevant sources. This would incorporate the cost of supplies, staff, and medications for the provider; the costs to the payer which reimburses a wide variety of providers across multiple settings; and also the costs to patients and families in the form of out-of-pocket monetary costs as well as the value of time spent providing informal care. Yet the evidence base on palliative care is overwhelmingly reliant on routinely collected hospital or payer data—rarely both together—and rarely inclusive of patient and family costs. Consequently little is understood about palliative care’s impact on overall costs.
For example, hospital palliative care reduces costs in part by expediting discharge (12), but this may pass costs on to other parts of the formal health system (such as skilled nursing facilities, home health agencies, or hospice), and/or onto informal carers. By not measuring patients’ utilization of all modalities and settings of care, researchers also fail to appreciate that these other modalities may be contributing to the positive effects attributed to palliative care. That is, a palliative care intervention may reduce future hospital costs in part by leading to hospice care in the home; not only is hospice a source of costs to the payer, but it is also an intervention that is known to prevent hospital costs. The societal perspective allows for testing between cost-shifting and overall cost-savings, and also for exploring potentially additive effects of multiple interventions.
A third important issue in health economic studies of palliative care is the time window of analysis. This is particularly obvious in studies that focus only on single-index hospital admissions, which are demonstrably unrepresentative of care for serious chronic illnesses and complex (multi-morbid) conditions. The most popular approach used to examine longer timeframes is the decedent cohort study, but this approach carries its own subtle complications. Both the value (13) and the limitations (14) of decedent cohort designs in general have been well debated previously. But there is a specific risk of bias in economic studies because costs add up over time. In the cost analysis of the landmark RCT by Temel and colleagues (15), utilization was lower among PC patients both immediately after diagnosis and in the last weeks of life, but mean total costs were higher in the PC group due to the positive survival effect (16). The extra costs associated with longer life eclipsed the savings from lower intensity treatment—the PC intervention was highly effective but did not decrease costs at the patient level from intervention (or diagnosis) to death. Total resource use is the most important outcome of interest in economics and policy, but this can only be an outcome in decedent cohort studies on an assumption of zero mortality effect in either direction.
These limitations are important, and they are not unjustified or easily avoided. The practical and ethical challenges in conducting primary research on seriously ill populations are well established. Current evidence focuses on hospital and end-of-life phases because these costs are routinely collected by providers and payers. Moreover, these are the highest-cost episodes and phases of care. Hospital costs are the main component of end-of-life care costs (17), and up to one-half of people worldwide die in hospitals (18,19). Cost-savings achieved in these episodes are not trivial, even if this is a narrow approach. Similarly the lack of evidence on outcomes in economic studies reflects profound methodological difficulties in recruitment and measurement (20-22).
Proposed strategies to overcome current limitations
While these limitations are understandable, it is nevertheless essential that they are tackled. There is no single, perfect solution. Instead we offer a set of recommendations that together should improve on the current situation. These have both to do with adequate collection of data, as well as combination of data sources and types.
Filling the gaps
New primary research that collects original data in the domains currently lacking is an obvious starting point. In settings where cost data are automatically tabulated in administrative databases, the most crucial data to collect prospectively and in standardized fashion are the clinical and patient-reported outcomes of interest, which may include patient-reported costs. In prospective trials of palliative care interventions, the most likely data gap is for healthcare utilization and costs, including those outside of hospital settings. In administrative datasets in the US, one of the most glaring gaps is the lack of a method for systematically coding palliative care itself in administrative data. The most widely used healthcare coding system globally is the International Classification of Diseases (ICD-10), which does have a “palliative care encounter” code, but this is defined as comfort care intent, and not necessarily the involvement of palliative care specialists. As a result this code may have low sensitivity and low specificity for palliative care specialist encounters. This leaves a palliative care gap in many datasets that are otherwise quite comprehensive—for example, the Health & Retirement Study offers large, rich, population-representative data on a wide range of health and social factors (23) but does not include specialist palliative care use or access.
Connecting the islands
If clinical and patient-reported outcomes are routinely gathered, such as symptom scores, they are stored in electronic health records (EHR) systems. Records of palliative care encounters may be stored in those systems too, or in free-standing databases, or in creative uses of billing and administrative data systems. Those sources tend to be owned by institutions such as hospital systems and provider groups. In contrast payers tend to be the entities that have records of healthcare utilization across settings and providers. Health departments are the definitive sources of information about the dates and causes of death as recorded on death certificates.
Bridging all of these islands of data is a pre-requisite for conducting large-scale health services research in this area, which entails negotiating among various data owners. Theoretically any prospective palliative care study that collects patient-reported outcomes and palliative care encounters as primary data could access all of the other necessary data points at a later date, retrospectively. Some funding agencies encourage researchers to submit proposals for supplemental funding, which could be crucial in such a scenario. But if patient-reported outcomes are not routinely measured and documented electronically in the population of interest (both palliative care recipients and non-recipients alike), that gap cannot be filled retrospectively.
Piecing evidence together
Drawing on multiple sources of published data, routinely collected and longitudinal data may also make it possible to model the effects of access to palliative care on the basis of need to give policymakers the most realistic and rigorous possible evidence. This, too, may require the combination of institutional (providers, payers), public health data (disease registries and death registries), and census data (population characteristics) with meta-analyses of published research. This will require cross-sector collaboration that may be unfamiliar to some researchers.
Conclusions
In summary, we can achieve a more comprehensive approach to economic outcomes measurement by assessing effects on both costs and outcomes; broadening the perspective on costs considerably; and overcoming data gaps and linking datasets. It may be that future evidence places routine palliative care provision in the north-east quadrant of Figure 1—not as simplistically dominant as is sometimes argued, but a strategy that under the right circumstances improves outcomes for populations with serious and complex needs, and without requiring the vast new resources associated with cost growth in other areas of healthcare.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith S, Brick A, O'Hara S, et al. Evidence on the cost and cost-effectiveness of palliative care: a literature review. Palliat Med 2014;28:130-50. [Crossref] [PubMed]
- Langton JM, Blanch B, Drew AK, et al. Retrospective studies of end-of-life resource utilization and costs in cancer care using health administrative data: a systematic review. Palliat Med 2014;28:1167-96. [Crossref] [PubMed]
- May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med 2014;17:1054-63. [Crossref] [PubMed]
- May P, Garrido MM, Cassel JB, et al. Using Length of Stay to Control for Unobserved Heterogeneity When Estimating Treatment Effect on Hospital Costs with Observational Data: Issues of Reliability, Robustness, and Usefulness. Health Serv Res 2016;51:2020-43. [Crossref] [PubMed]
- Khandelwal N, Kross EK, Engelberg RA, et al. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med 2015;43:1102-11. [Crossref] [PubMed]
- Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Re 2013;(6):CD007760.
- Gardiner C, Brereton L, Frey R, et al. Exploring the financial impact of caring for family members receiving palliative and end-of-life care: a systematic review of the literature. Palliat Med 2014;28:375-90. [Crossref] [PubMed]
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes (4th edition), 2015.
- Neumann PJ, Palmer JA, Daniels N, et al. A strategic plan for integrating cost-effectiveness analysis into the US healthcare system. Am J Manag Care 2008;14:185-8. [PubMed]
- Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA 2016;316:2104-14. [Crossref] [PubMed]
- Farquhar M, Preston N, Evans CJ, et al. Mixed methods research in the development and evaluation of complex interventions in palliative and end-of-life care: report on the MORECare consensus exercise. J Palliat Med 2013;16:1550-60. [Crossref] [PubMed]
- May P, Garrido MM, Cassel JB, et al. Cost analysis of a prospective multi-site cohort study of palliative care consultation teams for adults with advanced cancer: Where do cost-savings come from? Palliat Med 2017;31:378-86. [Crossref] [PubMed]
- Earle CC, Ayanian JZ. Looking back from death: the value of retrospective studies of end-of-life care. J Clin Oncol 2006;24:838-40. [Crossref] [PubMed]
- Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA 2004;292:2765-70. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Greer JA, Jackson VA, Meier DE, et al. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin 2013;63:349-63. [Crossref] [PubMed]
- Simoens S, Kutten B, Keirse E, et al. The costs of treating terminal patients. J Pain Symptom Manage 2010;40:436-48. [Crossref] [PubMed]
- Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of Site of Death, Health Care Utilization, and Hospital Expenditures for Patients Dying With Cancer in 7 Developed Countries. JAMA 2016;315:272-83. [Crossref] [PubMed]
- Broad JB, Gott M, Kim H, et al. Where do people die? An international comparison of the percentage of deaths occurring in hospital and residential aged care settings in 45 populations, using published and available statistics. Int J Public Health 2013;58:257-67. [Crossref] [PubMed]
- Higginson IJ, Evans CJ, Grande G, et al. Evaluating complex interventions in End of Life Care: the MORECare Statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11:111. [Crossref] [PubMed]
- Normand C. Measuring outcomes in palliative care: limitations of QALYs and the road to PalYs. J Pain Symptom Manage 2009;38:27-31. [Crossref] [PubMed]
- Round J. Is a QALY still a QALY at the end of life? J Health Econ 2012;31:521-7. [Crossref] [PubMed]
- Program on Global Aging, Health, and Policy. The Gateway to Global Aging Data. 2018; Available online: https://g2aging.org/, accessed January 25th, 2018.