Cannabis in palliative care: current challenges and practical recommendations
IntroductionOther Section
- Introduction
- Current challenges when considering CBM in palliative care
- Practical recommendations and clinical pearls
- Conclusions
- Acknowledgements
- Footnote
- References
The need for palliative care is increasing at a rapid pace in the context of an aging population, and where 75% of deaths are caused by chronic and progressive conditions (1). Generally, subjects with terminal illnesses experience significant symptom burden that often increases in intensity over time. In cross-sectional studies, patients report 8–12 symptoms, with fatigue, pain, anorexia, cachexia, dyspnea, anxiety, and depression being particularly common (2,3).
There are many opportunities to improve palliative care by utilizing both pharmacological and nonpharmacological means. Poor symptom control and/or intolerable adverse effects attributed to opioids and other medications encourage the search for other therapeutic strategies, such as cannabinoid-based medicines (CBM). These include approved pharmaceutical cannabinoids such as Nabilone (Cesamet®), Nabiximols (Sativex®), Dronabinol (Marinol®—no longer available in Canada) and medical cannabis products such as dried flowers or edible oils.
Integration of CBM into palliative care has been delayed by many obstacles, including a paucity of clinical research data, poor clinical knowledge on how to initiate and monitor cannabinoid treatments, and conflicting or confusing regulatory frameworks. This situation is further complicated by political and public opinions that either stigmatize cannabis use or claim cannabinoids of various formulations are highly effective in palliative care and for a range of other conditions. Furthermore, a survey published in 2017 of adult cancer patients at a major cancer center in Seattle, WA found high rates of active cannabis use (24% in the last year) and also showed that cancer patients desire but are not receiving information about cannabis from oncology healthcare providers (4). Interestingly, a more recent survey of 237 U.S. oncologists published in May 2018 showed that while only 30% felt sufficiently informed to make recommendations regarding CBM, 80% of oncologists conducted discussions about CBM with their patients and 46% recommended CBM clinically. Additionally, 67% viewed it as a helpful adjunct to standard pain management strategies, and 65% thought CBM were equally or more effective than standard treatments for anorexia and cachexia (5). Meanwhile, the Dutch government recently agreed to fully reimburse medical cannabis for terminally ill patients, beginning in January 2019 (6).
It is in this context that we have addressed these challenges through consensus from expert opinion and a review of the literature and organized them to reflect the patient consultation process.
Thus, this paper will:
- Review the current challenges when considering CBM in palliative care;
- Provide a brief overview of the current general knowledge of cannabis and cannabinoids in reference to these specific challenges; and
- Offer practical recommendations and clinical pearls regarding appropriate and supportive use of CBM in palliative care.
Current challenges when considering CBM in palliative careOther Section
- Introduction
- Current challenges when considering CBM in palliative care
- Practical recommendations and clinical pearls
- Conclusions
- Acknowledgements
- Footnote
- References
Challenge 1: when is it appropriate to consider medical cannabis treatment for a palliative care patient?
Before considering the use of medical cannabis in palliative care, good clinical judgment should always determine if the timing and the indications for introducing this treatment are appropriate. For instance, it is essential to determine if there will be sufficient time to assess the potential therapeutic benefits of the cannabinoid treatment. Furthermore, in the terminal stages of cancer, delirium is a common finding and this could be exacerbated by the use of CBM.
Systematic reviews regarding the benefits of CBM for the management of pain reveal mixed recommendations (7-10). A recent review which aimed to assess the efficacy of CBM for relieving pain in patients with malignant disease demonstrated a significant analgesic effect in 15 of 18 trials as compared to placebo (11). However, a recent review from the College of Family Physicians of Canada (CFPC) recommended against the use of CBM as first or second line treatment to palliate cancer pain (strong recommendation) (12). According to the CFPC, clinicians could consider CBM for refractory cancer pain only after the following considerations have been met:
- Discussing the risks and benefits of CBM with patients;
- Patients have had a reasonable therapeutic trial of more than two prescribed analgesics and have persistent problematic pain despite optimized analgesic therapy;
- CBM are adjuncts to other prescribed analgesics.
The CFPC also recommends the approved CBM Nabilone or Nabiximols as the initial agents (strong recommendation), though only the latter has the indication for cancer pain by Health Canada.
While it is correct to argue that the effectiveness of CBM in treating pain in palliative care settings has not yet been well established in comparison to other therapies, the position of the CFPC is debatable for several reasons.
Despite the fact that most patients medicating with cannabis do so to reduce pain, a recent Israeli study on cannabis use in over 3,000 cancer patients showed a significant improvement in the control of other common symptoms, including sleep problems (70.8%), fatigue (55.9%), anxiety and depression (74.1%), and nausea and vomiting (54.7%). Only 18.7% of patients reported good quality of life prior to treatment initiation, while 69.5% reported good quality of life at 6 months. Furthermore, 36% of patients stopped using opioids and less than 20% discontinued their cannabis treatment. Of these, only 19.3% stopped due to side effects (13). Thus, the clinical usefulness of CBM, still considered by many to be limited to pain control, appears to encompass a much broader range of symptoms encountered in palliative care settings. Considering these recent findings, now may be time to re-examine not only the role of CBM in symptom control, but also whether these compounds should be offered earlier in the course of a comprehensive palliative care strategy, particularly for patients who have had prior positive experience regarding the alleviation of symptoms other than pain.
Furthermore, if CBM were to be considered, we call into question as to whether the recommended CBM Nabilone and Nabiximols should be used as first line agents. Nabilone is a synthetic tetrahydrocannabinol (THC) analogue in oral form that is 10 times more potent than natural THC. It is approved for chemotherapy-induced nausea and vomiting and has been used off label for pain (14-16). Since it is often reimbursed by public and private insurance plans (at least in Canada), an initial trial with this product could reasonably be considered. However, this is not necessarily the case with Nabiximols, a whole plant extract from Cannabis sativa in the form of an oromucosal spray with a 1:1 ratio of THC and cannabidiol (CBD). In Canada, it is listed for the management of cancer pain, neuropathic pain and spasticity in multiple sclerosis (17,18). Although the purity and potency of unregulated cannabis products may often be unreliable or inaccurately labeled when compared with Nabiximols, Canadian law requires that medical cannabis provided by Licensed Producers must comply with many of the same standards expected from the pharmaceutical industry. Consequently, many available products from Licensed Producers exhibit a potency of the active cannabinoid compounds THC and CBD that are similar to Nabiximols. Since these are the two most abundant cannabinoids found in cannabis, and in all likelihood responsible for most of the primary therapeutic benefits, it may be surmised that dose-equivalent effects should be expected when using similar administration routes. Furthermore, since Nabiximols is seldom reimbursed and can often exceed the cost of a similar medical cannabis oil product by 80% or more, it is unclear why clinicians should impose this financial burden on their patients.
Finally, one could also argue against the use of unapproved cannabinoids on the grounds that official guidelines regarding the appropriate dispensing of these medical cannabis products have not been issued, leaving clinicians with little instruction on route of administration, dosage, titration and monitoring. However, considering the striking similarities between these products and the approved pharmaceutical forms of cannabinoid Nabiximols, it seems logical that comparable guidelines should be obtainable.
Challenge 2: how do we define the objectives of cannabinoid therapy and manage expectations?
Defining clear clinical objectives with patients and their families is of great importance in palliative care. As discussed earlier, the focus is often on the treatment of cancer pain, but many patients may want to address other common symptoms at end of life, such as anxiety, depression, nausea, anorexia or insomnia, which might also be relieved by CBM. Others may be seeking for a reduction or cessation of certain medications, and particularly for the opioid-sparing effects of CBM that have been observed in preclinical and early clinical studies (19). Furthermore, some evidence of potential synergistic relief of pain with concomitant use of opioids has been demonstrated without significantly altering plasma opioid levels (20). It is crucial to address these expectations and explain that individual responses to CBM can vary considerably. Some symptoms, including neuropathic pain, nausea and muscle spasms, have been studied in larger clinical trials, and consequently patients should be reminded that the evidence for the treatment of other symptoms is still inconclusive.
In cases where patients expect medical cannabis to act as a curative strategy for their advanced health condition, this delicate discussion leads into a topic in palliative care that bears mention: facilitation of a patient’s right to access experimental treatments in line with their wishes and beliefs in the service of hope (21).
Preclinical evidence and a few case study reports have shown that cannabinoids might have disease-modifying effects. It is therefore not surprising that interest in using cannabis preparations to treat cancer has surged among patients and families. Several preclinical studies have demonstrated anti-tumor activity in cell cultures or animal models (22,23). In breast cancer, for example, cannabinoids have been shown in vitro to interact with multidrug resistant proteins, improving the effectiveness of antineoplastic medications (24), and emerging data has also shown that cannabis and cannabinoids do not negatively interact with presently available chemotherapeutics (25).
However, there are no phase II or III clinical trials large enough to provide the necessary evidence to support the disease-modifying effects of medical cannabis with regards to cancer. Some believe this indication generally requires a 10-fold dose increase of cannabinoids normally used for symptom control (26), which may provoke significant side effects, though a recent phase II trial using Nabiximols in glioblastoma has shown promising results using lower doses of cannabinoids (27). There are also important financial considerations when purchasing much higher doses of concentrated cannabis oil extracts (i.e., “Rick Simpson oil” or “Phoenix Tears”), not available through Health Canada approved Licensed Producers. In addition, basic safety issues must be addressed regarding the use of any cannabis products, particularly if derived from petroleum-based solvents or containing other contaminants. If such a trial is to be pursued by the patient, it may require support from a healthcare provider experienced in CBM and should be conducted in close collaboration with the oncology healthcare team.
However, the patient and family should be reminded that a realistically achievable outcome of medical cannabis treatments is to potentially better cope with the emotional, existential or spiritual suffering associated with distressing physical symptoms or particularly complex psychosocial situations at the end of life.
Challenge 3: is there a difference between a naïve versus an experienced cannabis user?
It is essential to determine if the patient is naïve to cannabis or has had prior experience, as high tolerance to many of the psychoactive effects of cannabis has been documented in chronic heavy users, presumably due to CB1 receptor downregulation by THC (28). It also appears that the subjective “high” or euphoric effect of THC usually occurs at higher doses than necessary for pain control (29).
It is also likely that patients using cannabis from illicit sources may be unaware of the concentration of THC and CBD in the products that they have used, and a cautious titration period with approved medical cannabis should be employed, even with experienced users, in order to determine the optimal dose. However, therapeutic and/or side effects for experienced patients are often more predictable based on their previous experience.
For patients naïve to cannabis, it is preferable to start with the lowest possible dose and follow the general rule: “start low, go up slow and stay low” (30). For patients with contraindications to THC or who may be apprehensive or sensitive to its specific psychoactive effects, an initial trial of CBD rich formulations may also be considered, as these compounds are generally better tolerated. Otherwise, when starting with formulations containing THC, slow dose escalation will decrease the likelihood of side effects. For those patients who are already using high doses of cannabis (i.e., >3 g/day) and whose survival is estimated to be of several months or more, harm-reduction strategies should be considered, similar to the precautions used with opioids. For these patients, longer acting preparations (i.e., cannabis oils and Nabilone) should be recommended. This reduces the triggering of the pleasure reward pathway, which is typically encountered with the use of high potency, short-acting formulations (i.e., flowers administered through inhalation via smoking and vaping) and is associated with psychological dependence and chemical coping conditions. Furthermore, the concomitant use of CBD, which does not produce the psychoactive effects unique to THC, should be also encouraged as a harm reduction strategy.
Challenge 4: which precautions and/or contraindications should be considered before authorizing medical cannabis in the palliative care population?
In general, cannabis is a safe product. A prospective Canadian cohort study showed there was no difference in the risk of developing serious adverse events among patients receiving a standardized herbal cannabis product (12.5% THC) for a 1-year period, when compared to controls (31). However, medical cannabis users (median cannabis dose of 2.5 g/day) were at increased risk of mild to moderate non-serious adverse events (i.e., not impacting overall function or requiring discontinuation of medical cannabis), which is consistent with a previous systematic review (32). A more recent meta-analysis and systematic review concluded, however, that cannabinoids were associated with an increased risk of short-term adverse events (7).
THC side effects
- Drowsiness, dizziness, dry mouth, anxiety, euphoria, paranoia, toxic psychosis, tachycardia, orthostatic hypotension, slowed reaction time, headache, blurred vision, cognitive impairment, and depression. Cannabinoid hyperemesis syndrome (CHS) is a rare side effect of unclear origin, with roughly 80 reported cases, mostly in chronic cannabis users (33).
CBD side effects
- With standard dosing: dry mouth, drowsiness, light headedness, hypotension, fatigue.
- At high doses (20 mg/kg): diarrhea, vomiting, fatigue, pyrexia, somnolence, and abnormal results on liver-function tests (34,35).
According to Health Canada, the contraindications that apply to those considering using prescription cannabinoid-based therapies, such as Nabilone, Nabiximols or Dronabinol, also apply to those considering using medical cannabis (36). These precautions are generally well known and may be appropriate in a younger or otherwise functional patient population. However, a strict interpretation may be questionable in palliative care settings and we will briefly discuss the most relevant items here.
Cannabis should be used with caution in patients with severe cardiac or pulmonary disease due to occasional hypotension (or possible hypertension), reflex tachycardia and syncope caused by THC-rich products. To date, however, CBM have not been implicated in QT/QTc interval prolongation (37). In patients with significant hepatic or renal impairment, no specific studies have been done with CBM, though it can be expected that effects would be more exaggerated or prolonged in these patients. Given that cannabinoids are highly protein-bound in the plasma, it is unlikely they will be removed by hemodialysis (21). Concomitant use of sedative-hypnotics, opioids or other psychoactive drugs may have additive or synergistic CNS-depressive or psychoactive effects. Cannabis should also be avoided in patients with a personal or family history of psychotic disorders, although emerging data seems to indicate that CBD-rich formulations may be a safer alternative as they have antipsychotic and anxiolytic properties (38-40).
Challenge 5: what is clinically relevant from the scientific literature on the pharmacology of cannabinoids, including metabolism and potential for drug-drug interactions?
There are several published articles describing the pharmacological characteristics of the cannabis plant (41-44), which contains up to 545 chemical compounds, 114 of which are unique phytochemicals called cannabinoids that interact with the endocannabinoid system (ECS) (45). The remainder includes over 200 terpenoids, that give cannabis its characteristic odour, flavonoids, fatty acids, among others—all with potential medical uses. Some terpenoids have even been shown to bind with cannabinoid receptors and are particularly targeted as having possible synergistic or “entourage effects”, though there is still little evidence to suggest a clear influence from these secondary compounds (46-48). Table 1 lists the possible therapeutic effects of the most commonly found cannabis terpenoids.

Full table
So far, studies have demonstrated efficacy of THC and CBD (41). THC binds as a partial agonist to G-protein-coupled cannabinoid receptors CB1 and CB2. It is thought to be responsible for most of the therapeutic effects attributed to cannabis, including mitigating pain, spasticity, nausea, insomnia and appetite loss. THC is also responsible for the unique psychoactive effects of cannabis through its actions at the CB1 receptor (49,50). These effects, which have been shown to be dose-related, can include distressing symptoms such as paranoia and anxiety, but may also produce euphoria or relaxation (30).
CBD, on the other hand, is generally well tolerated and does not appear to bind to either CB1 or CB2 receptors at physiologically meaningful concentrations, thereby averting the THC-mediated psychoactive effects (51). Results from preclinical studies suggest CBD has anti-inflammatory, analgesic, anti-nausea, anti-emetic, anti-psychotic, anti-ischemic, anxiolytic, and anti-epileptic effects (14). When used exclusively in certain conditions such as epilepsy and anxiety, large doses of CBD are often necessary (39,52-55). At lower doses, however, CBD may improve the tolerability and safety of THC by reducing many of the unwanted side effects (e.g., cognitive impairment, anxiety, paranoia, tachycardia) (15). Cannabinoids other than THC and CBD have yet to be adequately researched.
The metabolism of THC and CBD is not yet completely understood. It is carried out by the cytochrome P450 system (41), producing considerable variance in pharmacokinetics between the oral and inhaled route (see Table 2) (41,42,56). The CYP2C9 enzyme is thought to be responsible for the first-pass metabolism of THC. Another possible metabolic pathway for THC is through CYP3A4 enzymes. Interaction with potent CYP3A4 inhibitors, such as protease inhibitors, clarithromycin, ketoconazole, sildenafil and warfarin have been mentioned in the literature, but only as isolated case reports and do not seem to be clinically relevant (57-59).
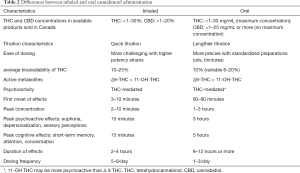
Full table
Evidence for potential interaction between pharmaceutical CBD formulations (5–50 mg/kg/day) and antiepileptic drugs in adults and children has also been reported (52), and monitoring levels of clobazam and N-desmethylclobazam has been recommended (35,53,60).
Challenge 6: what are the important considerations when selecting the appropriate medical cannabis strain and THC:CBD ratio?
Strain selection is one of the most perplexing issues regarding medical cannabis, since the commonly used but inaccurate vernacular system of distinguishing sativa and indica types of cannabis has developed independent of scientific and taxonomic classification systems. Although efforts are ongoing to adopt a more reliable system of science-based “chemovar” classification, there is little data to suggest specific therapeutic effects of the different cannabis strains (61). Small studies and anecdotal reports have suggested that sativa-dominant strains are characterized as uplifting and energetic, giving a feeling of optimism and well-being (62), while indica-dominant strains are described as calming and grounding and are said to result in relaxation, stress relief, and an overall sense of serenity (63). While this debate will surely continue for some time, there is little doubt that there is a significant overlap in therapeutic effects among the 700 or more cannabis varieties in existence (64).
Standardized testing of THC and CBD content in regulated medical cannabis products has been established in a few countries, including Canada. However, many other jurisdictions still face inconsistent labeling information. Furthermore, a genetic analysis of 81 cannabis samples issued from licensed producers in Canada found that in 6 of 17 comparisons (35%), samples were more genetically similar to samples with different names than to samples with identical names (65). Consequently, the genetic identity of a cannabis strain cannot be inferred by its name or by its reported ancestry (66).
In summary, until standardized formulations become available, patients should focus primarily on THC and CBD. They should also be encouraged to try different chemovars with similar THC:CBD ratios and keep a detailed journal documenting their personal responses. While there seems to be a growing consensus regarding the benefits of using CBD with THC, much speculation remains about the ideal ratio that would optimize tolerability and efficacy. However, over 200 studies undertaken with Nabiximols has shown that a 1:1 ratio is usually well tolerated.
Challenge 7: which method of administration of CBM will be best for a palliative care patient?
CBM may be administered in many forms, either through inhalation, oral preparations, oromucosal sprays, rectal suppositories, salves and topically delivered preparations. Other formulations such as high-potency concentrates and innovative delivery devices offering more accurate dosing (i.e., Syqe Medical™ inhaler, hmbldt™ dose pen) may be available to healthcare practitioners, according to local regulations (67).
Inhalation either by smoking combusted plant material or vaporization remain popular routes of administration, as the effects are quickly experienced, making titration easier. Though a large retrospective study found no association between cannabis smoking and lung cancer (68,69), smoking should be discouraged due to the obvious risks of bronchial inflammation (70,71). Patients can also be reminded that there is a significant loss through side stream combustion, which has been evaluated at approximately 50% of the THC content. Of the remaining inhaled smoke, another 50% is exhaled again, with some of the remaining smoke undergoing localized metabolism in the lung (70).
Vaporization of cannabis is becoming increasingly popular among medical cannabis users due to its perceived harm reduction through the release of a significantly lower percentage of noxious chemicals (42,72,73). When cannabis is given through this route, it vaporizes at a much lower temperature (175–225 degrees Celsius), and aerosolized active components can be inhaled without the generation of smoke (74), with significantly lower odor and carbon monoxide levels (75).
Orally administered cannabis extracts offer the advantage of more precise dosing, but many factors influence the time of onset, duration and intensity of effects. For example, hepatic first-pass metabolism transforms much of the Δ9 THC into 11-OH THC, a possibly more potent form, thus requiring a low starting dose and careful titration.
Table 2 indicates the differences between inhaled and oral routes of administration (41,42,56).
Table 3 summarizes the more relevant evidence regarding the use of cannabinoids for palliative care symptoms. In addition, suggestions for the type of cannabinoid and the most effective route of administration are also included, based on clinical experience and previous published evidence (96).
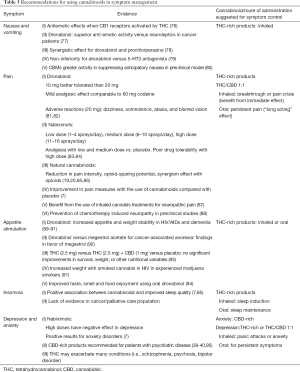
Full table
Challenge 8: what is considered a safe approach for dose initiation and titration?
Exact doses vary widely and depend upon individual patient need and tolerance of side effects. Furthermore, THC is also considered to have a wide safety margin, where non-lethal oral doses of up to 3,000 mg/kg have been observed in monkeys (97). Consequently, some patients will unintentionally overload their system and expose themselves to unwanted side effects and increased tolerance. Many cannabis-naïve patients will experience adverse events with a starting dose as low as 5 or 10 mg of THC. A recent trial assessed the dose-related effects of THC on emotional responses to acute psychosocial stress. In this study, a dose of 7.5 mg of THC dampened negative emotional responses without influencing performance while 12.5 mg resulted in a slight but significantly increased negative affect overall (98).
An often referenced study involving titration protocols in cancer pain by Johnson et al. using Nabiximols vs THC or placebo, showed that 43% of patients taking the THC:CBD extract Nabiximols achieved a 30% or greater improvement in their pain score at a median dose of 8.75 sprays per day (≥25 mg of THC per day) (84). Another study on chronic neuropathic pain showed that a single inhalation of 25 mg of herbal cannabis containing 9.4% THC (equal to 2.5 mg of THC) TID for 5 days reduced the intensity of pain, improved sleep and was well tolerated (29).
Therefore, as these studies suggest, many experts now believe that the threshold for the medical benefits of THC is far lower than previously thought. A sub-psychoactive dose as little as 2.5 mg of THC or less, with or without CBD, may offer many of the therapeutic benefits of cannabis, while avoiding intoxication. Moreover, the different pharmacokinetics between inhaled and oral routes may not play a significant role in the overall effects at this starting dose (99). Patients can maintain this dose for 2–3 days and then titrate accordingly.
Regarding CBD-rich cannabis dosing, some authors recommend starting with lower doses than those seen in clinical studies with CBD isolate, starting with 5–20 mg CBD per day of oral preparations, in two or three divided doses (30).
Challenge 9: can some of the psychoactive effects of cannabis be beneficial in palliative care?
Cannabinoids may produce unique effects, which were known primarily through traditional medicinal and cultural uses and in anecdotal reports from patients and caregivers (21). These effects include euphoria and relaxation, aversive memory extinction, increased focus of attention, enhanced sensory perception and introspective abilities, and temporary dissociative states (100). Understandably, practitioners may feel somewhat perplexed about how to best manage these unique properties often considered as the “intoxicating” effects of cannabis. We will briefly discuss the issues regarding the most common psychoactive effects associated with cannabis and encourage practitioners to engage in an open discussion with their patients in order to better understand the therapeutic aspects of their experiences.
Stimulation of cannabinergic activity in certain parts of the brain is known to play a key role in memory extinction of aversive memories and anxious thoughts or behaviours (101). A recent study also showed that chronic cannabis use is associated with blunted stress reactivity and lower cortisol levels when exposed to an acute stress test (102). There is also some evidence of the benefit of cannabinoid use in those with recognized post-traumatic stress disorder (PTSD) (103). Targeting the ECS may, therefore, offer potential benefit in reducing the psychological trauma associated with terminal illness diagnosis and invasive treatments.
Cannabis has long been used as an enhancer, heightening sensory perceptions and awareness, including increased appreciation of music, tastes, scents, or other aesthetic pleasures. It could also help to heighten awareness of moment-to-moment presence, a state that is all the more critical when one’s days are numbered. This could very well play an important therapeutic role for certain patients faced with the despair of a terminal illness, and the loss of function that typically accompanies it. The potential contribution to spiritual growth and development may help to create “a good death” or “tending to dignity by way of the senses” as stated by Dr. B. J. Miller (21).
When taken at much higher doses, cannabis is one of the many drugs that can induce temporary dissociative-like states, which can create a “distancing” from pain experience, without relieving it through a direct mechanism. Though many patients may not be receptive to these types of effects, some may consider them as a safer and more desirable option than opioids when overwhelmed with unbearable physical and or psychological symptoms, which can happen at the end of life.
Practical recommendations and clinical pearlsOther Section
- Introduction
- Current challenges when considering CBM in palliative care
- Practical recommendations and clinical pearls
- Conclusions
- Acknowledgements
- Footnote
- References
Common questions and recommendations to assist the palliative care or multidisciplinary team considering medical cannabis treatment are summarized in Table 4.
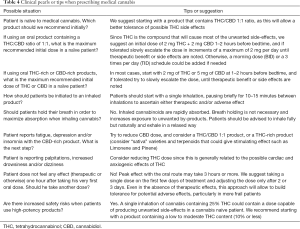
Full table
ConclusionsOther Section
- Introduction
- Current challenges when considering CBM in palliative care
- Practical recommendations and clinical pearls
- Conclusions
- Acknowledgements
- Footnote
- References
CBM will undoubtedly play a larger role in palliative care medicine in the years to come but there are still hurdles preventing a safe and unencumbered system of access for patients. One notable obstacle lies in the fact that CBM are still not considered as approved treatments for any condition for reasons aforementioned. Large scale randomized control trials, still considered the authoritative arbiter to prove medical efficacy, are largely inaccessible to cannabis researchers for a variety of legitimate reasons. While modern pharmaceutical companies can no longer rely on expert testimonials and case reports to make broader claims about newly synthesized products, the historically safe profile of the cannabis plant could make data from other clinical trials more admissible in order to formulate reliable clinical practice guidelines.
AcknowledgementsOther Section
- Introduction
- Current challenges when considering CBM in palliative care
- Practical recommendations and clinical pearls
- Conclusions
- Acknowledgements
- Footnote
- References
The authors wish to thank Devon Phillips, Director at the McGill Faculty of Medicine Palliative Care Program, for her generous time and guidance that lead to the realization of this document.
FootnoteOther Section
- Introduction
- Current challenges when considering CBM in palliative care
- Practical recommendations and clinical pearls
- Conclusions
- Acknowledgements
- Footnote
- References
Conflicts of Interest: Dr. C Cyr declares the following possible conflicts of interest: honorarium received from cannabis Licensed Producers (Tilray, Cannimed, Tweed) for continuing medical education (CME) talks. Medical advisor to InVentive/Shoppers Drug Mart. Dr. MF Arboleda declares the following possible conflicts of interest: McGill University Supportive Care and Medical Cannabis Post-Doctoral Research Fellow sponsored by Tetra Bio-Pharma Inc. Co-investigator for a Phase II and a Phase III clinical trial sponsored by Tetra Bio-Pharma Inc. Professor LG Balneaves declares the following conflicts of interest: Member, Conference Planning Committee of the Canadian Consortium for the Investigation of Cannabinoids. Dr. P Daeninck declares the following possible conflicts of interest: Scientific advisory board member for Canadian cannabis producers: ABcann Medicinals and Bonify, Medical advisor to InVentive/Shoppers Drug Mart, Medical advisor to Australian cannabis producer Little Green Pharma, Current President of the Board of Directors of the Canadian Consortium for the Investigation of Cannabinoids. A Néron declares having the following possible conflicts of interest: honorarium received from Canopy Growth Corporation as member of the scientific planning committee for Medical Cannabis Web-based education program for Pharmacists, Medical advisor to InVentive/Shoppers Drug Mart. E Prosk declares the following possible conflicts of interest: Director of Santé Cannabis, a medical clinic specializing in clinical research. Dr. A Vigano declares the following possible conflicts of interest: Research Director of Santé Cannabis, a medical clinic specializing in clinical research. Principal Investigator for a Phase II and a Phase III clinical trial sponsored by Tetra Bio-Pharma Inc. Dr. SK Aggarwal has no conflicts of interest to declare.
ReferencesOther Section
- Introduction
- Current challenges when considering CBM in palliative care
- Practical recommendations and clinical pearls
- Conclusions
- Acknowledgements
- Footnote
- References
- McNamara B, Rosenwax LK, Holman CDJ. A method for defining and estimating the palliative care population. J Pain Symptom Manage 2006;32:5-12. [Crossref] [PubMed]
- Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res 1994;3:183-9. [Crossref] [PubMed]
- Van Lancker A, Velghe A, Van Hecke A, et al. Prevalence of symptoms in older cancer patients receiving palliative care: a systematic review and meta-analysis. J Pain Symptom Manage 2014;47:90-104. [Crossref] [PubMed]
- Pergam SA, Woodfield MC, Lee CM, et al. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer 2017;123:4488-97. [Crossref] [PubMed]
- Braun IM, Wright A, Peteet J, et al. Medical Oncologists’ Beliefs, Practices, and Knowledge Regarding Marijuana Used Therapeutically: A Nationally Representative Survey Study. J Clin Oncol 2018;36:1957-1962. [Crossref] [PubMed]
- Government agrees to free medicinal cannabis for terminally-ill patients - The Post 2018. Available online: http://cphpost.dk/news/government-agrees-to-free-medicinal-cannabis-for-terminally-ill-patients.html, accessed April 18, 2018.
- Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015;313:2456-73. [Crossref] [PubMed]
- Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician 2018;64:e78-e94. [PubMed]
- Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018;3. [PubMed]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press, 2017.
- Darkovska-Serafimovska M, Serafimovska T, Arsova-Sarafinovska Z, et al. Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases. J Pain Res 2018;11:837-42. [Crossref] [PubMed]
- Allan GM, Ramji J, Perry D, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician 2018;64:111-20. [PubMed]
- Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med 2018;49:37-43. [Crossref] [PubMed]
- Turgeman I, Bar-Sela G. Cannabis Use in Palliative Oncology: A Review of the Evidence for Popular Indications. Isr Med Assoc J 2017;19:85-8. [PubMed]
- Murnion B. Medicinal cannabis. Aust Prescr 2015;38:212-5. [Crossref] [PubMed]
- Schrot RJ, Hubbard JR. Cannabinoids: Medical implications. Ann Med 2016;48:128-41. [Crossref] [PubMed]
- Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011;18:1122-31. [Crossref] [PubMed]
- Nurmikko TJ, Serpell MG, Hoggart B, et al. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 2007;133:210-20. [Crossref] [PubMed]
- Nielsen S, Sabioni P, Trigo JM, et al. Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis. Neuropsychopharmacology 2017;42:1752-65. [Crossref] [PubMed]
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 2011;90:844-51. [Crossref] [PubMed]
- Aggarwal SK. Use of cannabinoids in cancer care: palliative care. Curr Oncol 2016;23:S33-36. [PubMed]
- Pisanti S, Malfitano AM, Grimaldi C, et al. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract Res Clin Endocrinol Metab 2009;23:117-31. [Crossref] [PubMed]
- Holland ML, Lau DTT, Allen JD, et al. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br J Pharmacol 2007;152:815-24. [Crossref] [PubMed]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 1998;95:15665-70. [Crossref] [PubMed]
- Engels FK, de Jong FA, Sparreboom A, et al. Medicinal cannabis does not influence the clinical pharmacokinetics of irinotecan and docetaxel. Oncologist 2007;12:291-300. [Crossref] [PubMed]
- Phoenix Tears. Rick Simpson. 2014. Available online: http://phoenixtears.ca/, accessed February 9, 2018.
- ClinicalTrials.gov. A Safety Study of Sativex in Combination With Dose-intense Temozolomide in Patients With Recurrent Glioblastoma. 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01812603, accessed May 26, 2018.
- Babor TF, Mendelson JH, Greenberg I, et al. Marijuana consumption and tolerance to physiological and subjective effects. Arch Gen Psychiatry 1975;32:1548-52. [Crossref] [PubMed]
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010;182:E694-701. [Crossref] [PubMed]
- MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med 2018;49:12-9. [Crossref] [PubMed]
- Ware MA, Wang T, Shapiro S, et al. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain 2015;16:1233-42. [Crossref] [PubMed]
- Wang T, Collet JP, Shapiro S, et al. Adverse effects of medical cannabinoids: a systematic review. CMAJ 2008;178:1669-78. [Crossref] [PubMed]
- Sorensen CJ, DeSanto K, Borgelt L, et al. Cannabinoid Hyperemesis Syndrome: Diagnosis, Pathophysiology, and Treatment-a Systematic Review. J Med Toxicol 2017;13:71-87. [Crossref] [PubMed]
- Bergamaschi MM, Queiroz RH, Zuardi AW, et al. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf 2011;6:237-49. [Crossref] [PubMed]
- Devinsky O, Cross JH, Wright S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med 2017;377:699-700. [Crossref] [PubMed]
- Health Canada. Information for Health Care Professionals: Cannabis (marihuana, marijuana) and the cannabinoids. 2013. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-use-marijuana/information-medical-practitioners/information-health-care-professionals-cannabis-marihuana-marijuana-cannabinoids.html, accessed May 26, 2018.
- Sellers EM, Schoedel K, Bartlett C, et al. A Multiple-Dose, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group QT/QTc Study to Evaluate the Electrophysiologic Effects of THC/CBD Spray. Clin Pharmacol Drug Dev 2013;2:285-94. [Crossref] [PubMed]
- Deiana S. Medical use of cannabis. Cannabidiol: a new light for schizophrenia? Drug Test Anal 2013;5:46-51. [Crossref] [PubMed]
- Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front Pharmacol 2017;8:259. [Crossref] [PubMed]
- Hahn B. The Potential of Cannabidiol Treatment for Cannabis Users With Recent-Onset Psychosis. Schizophr Bull 2018;44:46-53. [Crossref] [PubMed]
- Abrams DI, Guzman M. Cannabis in cancer care. Clin Pharmacol Ther 2015;97:575-86. [Crossref] [PubMed]
- Borgelt LM, Franson KL, Nussbaum AM, et al. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013;33:195-209. [Crossref] [PubMed]
- Javid FA, Phillips RM, Afshinjavid S, et al. Cannabinoid pharmacology in cancer research: A new hope for cancer patients? Eur J Pharmacol 2016;775:1-14. [Crossref] [PubMed]
- Bridgeman MB, Abazia DT. Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting. P T 2017;42:180-8. [PubMed]
- Ahmed SA, Ross SA, Slade D, et al. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015;117:194-9. [Crossref] [PubMed]
- Beaulieu P, Boulanger A, Desroches J, et al. Medical cannabis: considerations for the anesthesiologist and pain physician. Can J Anaesth 2016;63:608-24. [Crossref] [PubMed]
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011;163:1344-64. [Crossref] [PubMed]
- Andre CM, Hausman JF, Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front Plant Sci 2016;7:19. [Crossref] [PubMed]
- Jensen B, Chen J, Furnish T, Wallace M. Medical Marijuana and Chronic Pain: a Review of Basic Science and Clinical Evidence. Curr Pain Headache Rep 2015;19:50. [Crossref] [PubMed]
- Abrams DI. Integrating cannabis into clinical cancer care. Curr Oncol 2016;23:S8-14. [PubMed]
- Russo EB. Cannabidiol Claims and Misconceptions. Trends Pharmacol Sci 2017;38:198-201. [Crossref] [PubMed]
- Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017;58:1586-92. [Crossref] [PubMed]
- Geffrey AL, Pollack SF, Bruno PL, et al. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015;56:1246-51. [Crossref] [PubMed]
- Hess EJ, Moody KA, Geffrey AL, et al. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 2016;57:1617-24. [Crossref] [PubMed]
- Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011;36:1219-26. [Crossref] [PubMed]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42:327-60. [Crossref] [PubMed]
- Sachse-Seeboth C, Pfeil J, Sehrt D, et al. Interindividual variation in the pharmacokinetics of Delta9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9. Clin Pharmacol Ther 2009;85:273-6. [Crossref] [PubMed]
- Lindsey WT, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse 2012;38:334-43. [Crossref] [PubMed]
- Grayson L, Vines B, Nichol K, et al. An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav Case Rep 2017;9:10-1. [Crossref] [PubMed]
- Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 2016;15:270-8. [Crossref] [PubMed]
- Brunt TM, van Genugten M, Höner-Snoeken K, et al. Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J Clin Psychopharmacol 2014;34:344-9. [Crossref] [PubMed]
- MedicalJane. About Cannabis Sativa. A Brief Overview of Cannabis Sativa Strains. 2013. Available online: https://www.medicaljane.com/2013/07/22/cannabis-sativa-as-explained-by-medical-jane/, accessed October 24, 2017.
- MedicalJane. About Cannabis Indica. A Brief Overview of Cannabis Indica Strains. 2013. Available online: https://www.medicaljane.com/2013/07/25/cannabis-indica-as-explained-by-medical-jane/, accessed October 24, 2017.
- Hazekamp A, Fischedick JT. Cannabis - from cultivar to chemovar. Drug Test Anal 2012;4:660-7. [Crossref] [PubMed]
- Sawler J, Stout JM, Gardner KM, et al. The Genetic Structure of Marijuana and Hemp. PLOS ONE 2015;10. [Crossref] [PubMed]
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 2006;66:234-46. [Crossref] [PubMed]
- Eisenberg E, Ogintz M, Almog S. The pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: a phase 1a study. J Pain Palliat Care Pharmacother 2014;28:216-25. [Crossref] [PubMed]
- Sidney S, Quesenberry CP, Friedman GD, et al. Marijuana use and cancer incidence (California, United States). Cancer Causes Control 1997;8:722-8. [Crossref] [PubMed]
- Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013;10:239-47. [Crossref] [PubMed]
- Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol 2014;46:65-81. [Crossref] [PubMed]
- Lee MHS, Hancox RJ. Effects of smoking cannabis on lung function. Expert Rev Respir Med 2011;5:537-46. [Crossref] [PubMed]
- Gieringer D, St. Laurent J, Goodrich S. Cannabis vaporizer combines efficient delivery of THC with effective suppression of pyrolytic compounds. J Cannabis Ther 2004;4:7-27. [Crossref]
- Tashkin DP. How beneficial is vaping cannabis to respiratory health compared to smoking? Addiction 2015;110:1706-7. [Crossref] [PubMed]
- Abrams DI, Vizoso HP, Shade SB, et al. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther 2007;82:572-8. [Crossref] [PubMed]
- Earleywine M, Barnwell SS. Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J 2007;4:11. [Crossref] [PubMed]
- Van Sickle MD, Oland LD, Ho W, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology 2001;121:767-74. [Crossref] [PubMed]
- Machado Rocha FC, Stéfano SC, De Cássia Haiek R, et al. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care (Engl) 2008;17:431-43. [Crossref] [PubMed]
- Lane M, Vogel CL, Ferguson J, et al. Dronabinol and prochlorperazine in combination for treatment of cancer chemotherapy-induced nausea and vomiting. J Pain Symptom Manage 1991;6:352-9. [Crossref] [PubMed]
- Meiri E, Jhangiani H, Vredenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin 2007;23:533-43. [Crossref] [PubMed]
- Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol 2011;163:1411-22. [Crossref] [PubMed]
- Noyes R, Brunk SF, Baram DA, et al. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol 1975;15:139-43. [Crossref] [PubMed]
- Noyes R, Brunk SF, Avery DA, et al. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther 1975;18:84-9. [Crossref] [PubMed]
- Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain 2012;13:438-49. [Crossref] [PubMed]
- Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010;39:167-79. [Crossref] [PubMed]
- Bar-Sela G, Vorobeichik M, Drawsheh S, et al. The medical necessity for medicinal cannabis: prospective, observational study evaluating the treatment in cancer patients on supportive or palliative care. Evid Based Complement Alternat Med 2013;2013. [Crossref] [PubMed]
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci 2004;74:1317-24. [Crossref] [PubMed]
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data. J Pain 2015;16:1221-32. [Crossref] [PubMed]
- Ward SJ, McAllister SD, Kawamura R, et al. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 2014;171:636-45. [Crossref] [PubMed]
- Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995;10:89-97. [Crossref] [PubMed]
- Volicer L, Stelly M, Morris J, et al. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 1997;12:913-9. [Crossref] [PubMed]
- Haney M, Rabkin J, Gunderson E, et al. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology 2005;181:170-8. [Crossref] [PubMed]
- Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002;20:567-73. [Crossref] [PubMed]
- Cannabis-In-Cachexia-Study-Group, Strasser F, Luftner D, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 2006;24:3394-400. [Crossref] [PubMed]
- Brisbois TD, de Kock IH, Watanabe SM, et al. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol 2011;22:2086-93. [Crossref] [PubMed]
- McLoughlin BC, Pushpa-Rajah JA, Gillies D, et al. Cannabis and schizophrenia. Cochrane Database Syst Rev 2014. [PubMed]
- Maida V, Daeninck PJ. A user’s guide to cannabinoid therapies in oncology. Curr Oncol 2016;23:398-406. [Crossref] [PubMed]
- Hartung B, Kauferstein S, Ritz-Timme S, et al. Sudden unexpected death under acute influence of cannabis. Forensic Sci Int 2014;237:e11-13. [Crossref] [PubMed]
- Childs E, Lutz JA, de Wit H. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug and Alcohol Dependence 2017;177:136-44. [Crossref] [PubMed]
- Russo EB. Current Therapeutic Cannabis Controversies and Clinical Trial Design Issues. Front Pharmacol 2016;7:309. [Crossref] [PubMed]
- Good MI. Substance-induced dissociative disorders and psychiatric nosology. J Clin Psychopharmacol 1989;9:88-93. [Crossref] [PubMed]
- Akirav I. Targeting the endocannabinoid system to treat haunting traumatic memories. Front Behav Neurosci 2013;7:124. [Crossref] [PubMed]
- Cuttler C, Spradlin A, Nusbaum AT, et al. Blunted stress reactivity in chronic cannabis users. Psychopharmacology 2017;234:2299-309. [Crossref] [PubMed]
- Jetly R, Heber A, Fraser G, et al. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015;51:585-8. [Crossref] [PubMed]


