A hunger for hunger: a review of palliative therapies for cancer-associated anorexia
Introduction
Many patients with advanced, incurable cancer suffer from loss of appetite, herein referred to as anorexia. The prevalence of this symptom varies from study to study. Reporting on data from the PreMiO Study that included 1,952 cancer patients, Muscaritoli and others noted that 40% of patients reported loss of appetite as per a validated questionnaire (1). Doherty and others described that among 221 patients with cancer or HIV infection in Bangladesh, loss of appetite was observed in only 24% (2). However, in most large studies that have examined symptom prevalence in patients with advanced cancer, loss of appetite occurs in the majority of patients. For example, Walsh and others followed 1,000 cancer patients in a prospective analysis of symptoms and observed that anorexia is experienced by well over half (3). This variability in accounting for symptom prevalence might be reflective of geographic and cultural variation in symptom reporting, variability among these studies with respect to where patients fall on their cancer trajectory (for example, patients early in their cancer journey are probably less likely to suffer from loss of appetite), and methods used to assess the presence of anorexia. Despite such variables, taken together, these studies suggest that loss of appetite is common among cancer patients.
What are the implications of loss of appetite in patients with cancer? Importantly, cancer-associated loss of appetite is not a simple, isolated symptom. It often occurs in the presence of a multiplicity of other symptoms like pain, fatigue, and weakness; however, even after accounting for the effects of other variables, the presence of cancer-associated anorexia was found to strongly influence patient satisfaction with their health and physical function (4). Surprisingly, despite the prevalence and distressing nature of this symptom, relatively little qualitative research has been undertaken to understand exactly how it is impacting patients and their families. Providing some of the most important work that has been published to date, Reid and others interviewed cancer patients and their families; their quotes indicate that this symptom is indeed troubling (5). One family member described, “At first I thought we were in limbo, nobody cared, that we couldn’t turn to anybody…nobody seemed to help us…we just had to cope on our own…at that particular time when (he) was just finding all this out; well, we were just being thrown in at the deep end. I thought that someone should have come and spoke to us as a family to tell us what to expect…when he wasn’t feeling well and he wasn’t eating…we didn’t know whether to call for a doctor or what or who to turn to.” A patient expressed frustration over lack of direction, “I was losing the weight and she (dietician) said ‘Oh well’ she says, ‘you don’t have to stick to that diet, you don’t need a diet…this business of the weight loss, it’s a, it’s a rare commodity that anybody knows all about it.” Such quotations that come directly from patients suggest that loss of appetite can be a frustrating symptom for patients and their family members. These powerful statements also show that those suffering from cancer-associated anorexia desire more information and direction from their healthcare providers.
This one symptom also has grave prognostic consequences. Quinten and others assembled data from 30 randomized trials from the European Organization for Research and Treatment (6). These patients had completed validated questionnaires on quality of life, including appetite at baseline. In a multivariate model of survival, clearly appetite loss provided important and statistically significant prognostic information that shows poor appetite is associated with poor survival (hazard ratio =1.05; 95% CI, 1.03–1.06; P<0.0001). Although it remains unclear whether reversal of anorexia translates into improvement in survival, importantly, these data show that palliating loss of appetite in patients with advanced cancer is an important end-of-life intervention that can potentially improve their quality of life. Indeed, one might argue that this line of thinking is critical to how we approach this symptom in the patient with advanced cancer and adds leverage in favor of the decision to palliate this symptom in advanced cancer patients.
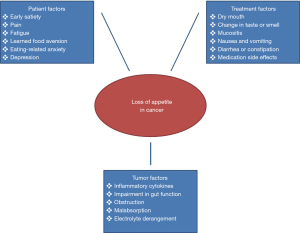
Based on the above, the objective of this review is to expound upon how best to treat advanced cancer patients who are suffering from cancer-associated loss of appetite. For all the reasons alluded to above—this symptom’s prevalence, its negative impact on quality of life, and its association with poor survival—all underscore the need to determine best measures to palliate this symptom. For the most part, we focus our discussion on the limitations of nutritional interventions and medications to improve appetite, but it is important to remember that cancer patients’ desire to eat is influenced by a multitude of factors (Figure 1), some of which are modifiable. For instance, tumor infiltration can directly impair function and motility of the gastrointestinal tract. Some tumors also produce substances that lead to early satiety. In these circumstances relief of any obstruction and anti-cancer therapy have the potential to improve appetite. Similarly, chemotherapy and radiation therapy may lessen a patient’s desire to eat by causing dry mouth, changes in taste or smell, mucositis, abdominal cramping, diarrhea or constipation, and severe nausea and vomiting; and commonly used medications such as opiates, cause gastrointestinal side effects that decrease one’s desire to eat. All this is to say that the whole picture must be considered when addressing cancer anorexia. Tumor and treatment-related barriers to adequate intake and desire to eat should be addressed in conjunction with nutritional interventions and appetite stimulants, when deemed appropriate.
Comments on invasive/parenteral nutrition support
When a healthcare provider first sees a cancer patient who is losing weight, who has no interest in food, and who describes a major loss of appetite, the initial response is to consider caloric repletion. Importantly, however, patients with cancer-associated anorexia and weight loss may appear to be starving, but in fact they are not (Table 1). Indeed, multiple studies have shown that in the setting of advanced incurable cancer, some forms of nutrition support can be detrimental. Nineteen randomized controlled studies were analyzed by the American Gastroenterological Association in a technical review (7). Meta-analysis of these oncologic trials showed no survival benefit from parenteral nutrition and, in actuality, harm. These studies showed that parenteral nutrition resulted in an increase in total complications (40% absolute risk difference; 95% CI, 14–66%) as well as infectious complications (16% absolute risk difference; 95% CI, 8–23%).

Full table
In concert with the above findings, several medical societies offer guidance on whether or not to prescribe nutrition support to patients with advanced cancer. The American Society for Parenteral and Enteral Nutrition (ASPEN) recommends nutrition support not be routinely used as an adjunct to chemotherapy or irradiation to the head and neck, abdomen, or pelvis (8). They also caution against the use of such nutrition support in terminally ill cancer patients. The European Society for Clinical Nutrition and Metabolism (ESPEN) acknowledges a large uncertainty about when to offer artificial nutrition in the context of cancer treatment (9). Their guidelines support artificial nutrition in cancer patients who are unable to maintain adequate oral intake (e.g., no food for more than 1 week or less than 60% of requirement for more than 1–2 weeks). They recommend escalation in the order of oral supplementation, followed by enteral nutrition, and only lastly, parenteral nutrition. The National Comprehensive Cancer Network guidelines in Palliative Care advise considering enteral and parenteral feeding (as appropriate) only when prognosis is longer than weeks to days (10). The decision to offer medically assisted nutrition remains complex and must be determined on a case-by-case basis after taking into account cancer type, treatment, anticipated duration and reversibility of nutritional deficit, prognosis, and patient preferences. For the most part, however, in patients with advanced, incurable cancer, the use of invasive nutrition support is strongly discouraged.
Comments on non-invasive nutrition support
Nutritional supplements
Although loss of appetite clearly constrains the ability of patients to eat, such challenging circumstances have not dissuaded healthcare providers from attempts to provide patients with specialized nutritional supplements with the goal of addressing the weight loss that many of these patients suffer.
First, amino acid supplements have been tested in several scenarios where patients experience decreased appetite, weight loss, and abnormal protein turnover including during wound healing, AIDS-associated wasting, and cancer-associated anorexia or weight loss (11-13). May and colleagues evaluated oral supplementation with a mixture of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine in patients with advanced cancer (14). The supplement was effective at increasing and maintaining free-fat mass for up to 24 weeks. Another study demonstrated that cancer patients show increased muscle protein synthesis following consumption of oral proteins enriched with 10% free leucine, an amino acid from which HMB is derived (15). With all the promise of the early studies, a phase III trial was conducted to assess the efficacy of HMB, arginine, and glutamine in increasing lean body mass of those with cachexia (16). Saliently, only 37% of participants in this trial completed the study protocol. Using an intention to treat analysis, no statistically significant difference was observed in lean body mass with the intervention. Importantly, the authors cited patients’ refusal to complete the study and gastrointestinal side effects as reasons for the high rate of non-compliance. This last point is an important one and emphasizes the challenges of providing oral nutritional supplements to cancer patients who are struggling to eat.
Amino acid derivatives have also been studied. Carnitine is a derivative of the amino acid lysine that is important in metabolism as it transports fatty acids across the mitochondrial membrane for oxidation and energy generation. Preclinical studies suggest that L-carnitine may have an application in the treatment of cancer associated loss of appetite and weight through regulation of inflammatory mediators like TNF alpha and IL-6 (17). Indeed, a randomized, placebo-controlled trial enrolled 72 patients with advanced pancreatic cancer and showed that carnitine supplementation improved body weight and some quality of life measures as compared to placebo (18). However, further study of this supplement is indicated prior to a recommendation that this amino acid derivative be used routinely. Yet another amino acid derivative that has undergone large-scale clinical testing is creatine, a supplement that appears to increase strength in healthy adults (19). However, a recent multi-institution, randomized controlled trial showed no statistically significant effect on weight gain, body composition, or quality of life (20).
Eicosapentaenoic acid (EPA) is another nutritional supplement which has been extensively studied. EPA is an omega-3 fatty acid found in fatty fish like mackerel, herring, and salmon. It is also a component of readily available over-the-counter fish oil preparations that are famed for their potent anti-inflammatory properties. In vitro and in vivo studies of fish oil seem promising, as omega-3 fatty acids influences host and tumor-produced mediators of inflammation (21). Further, EPA is thought to downregulate the ubiquitin-proteasome proteolytic pathway that is responsible for skeletal muscle loss in cancer-associated weight loss (22). However, several large phase III trials, which cumulatively included several hundred patients, have not shown that cancer patients acquire clinical benefits with this supplement (23-25). A Cochrane review of EPA for cancer-associated weight loss, most recently edited in 2017, concluded that there are insufficient data to support the use of fish oil in the management of cancer-associated anorexia and weight loss (26).
In general, if a cancer patient is most troubled by loss of appetite, it appears counterintuitive to attempt to treat this symptom complex with a nutritional supplement, largely because the anorexia itself serves as a barrier that makes it extremely challenging for patients to take the supplement. Indeed, the large number of negative trials reviewed above raises the question of whether these supplements truly do not work or whether they cannot work because patients are unable to tolerate them. In many respects, the answer to this question appears moot and suggests that other means of palliating symptoms and attempting to treat cancer-associated weight loss should be pursued.
Dietary counseling
Dietary counseling is often a component of a multimodal approach to cancer-associated weight loss and begins with a dietician’s formal assessment of the patient’s nutritional status. The goal of this assessment is to identify patient, cancer, and treatment-specific factors that are causing decreased dietary intake. Following this assessment, an individualized nutrition plan is sometimes developed to optimize caloric intake. Counseling interventions sometimes provide instruction on ways to fortify intake with such approaches as calorie dense foods and increased meal frequency.
While such dietary counseling can improve some metrics of nutritional intake, the current data do not show a consistent impact on traditional oncologic outcomes such as treatment response or survival. In one study, patients with solid organ malignancies receiving chemotherapy were randomized to twice weekly nutritional counseling versus ad-lib oral intake (27). Over the 5-month study period, dietary counseling resulted in increased energy intake, protein intake, and an insignificant increase in body weight without improving response rate or overall survival. Another study from Baldwin and others compared dietary counseling to ad lib intake for patients with advanced cancers and noted no differences in body weight or overall survival for the duration of intervention (28). For patients receiving chemotherapy, at least 3 randomized controlled trials evaluated the impact of dietary counseling-based nutritional intervention on quality of life and patient functional measures. None of these trials showed major benefit from dietary counseling (27-29). Finally, a meta-analysis demonstrated a statistically significant improvement in some quality of life function scales, symptom scales, and global quality of life with dietary counseling, but clinical and statistical heterogeneity limited the conclusions that can be drawn (30).
In the absence of a favorable impact on clinically important outcomes, what is the role of nutritional counseling? Interestingly, despite limited objective evidence many cancer patients who receive dietary counseling report higher perceived health benefits and overall satisfaction than patients receiving usual care (31). One participant noted, “I think everyone should receive advice from a dietician.” Another added, “I would like to thank the dietitian for (her) support—it really got me through the tough times of treatment.” These improvements in satisfaction scores likely reflect a mitigating effect that dieticians have on eating and weight loss in patients with advanced cancer (32). At the very least, however, the importance of showing a commitment to patients’ well-being and a demonstration of compassion when patients struggle with food intake appears enough to justify an ongoing multidisciplinary approach to cancer-associated anorexia with the inclusion of a dietician as an important part of that team.
Appetite stimulants
A subgroup of cancer patients who struggle with appetite loss will find an appetite stimulant helpful. Importantly, as far as we know, although appetite stimulants might improve appetite, they do not appear to improve global quality of life or survival; hence, their role should be clearly defined prior to prescribing them to patients. For practical purposes, the two classes of agents that have been most extensively and successfully studied are progestational agents and corticosteroids. Clinically, when patients desire an appetite stimulant, it appears most logical to consider prescribing an agent from one of these groups, as discussed further below.
Progestins
Of the appetite stimulants, megestrol acetate or other progestational agents are the most widely used and perhaps the best studied of all the orexigenic drugs. In a large, double-blind, placebo controlled trial from 1990, Loprinizi and colleagues demonstrated that megestrol acetate improved appetite among patients with advanced, hormonally insensitive cancer (33). Providing confirmatory evidence of appetite improvement, this study showed that 16% of patients who received megestrol acetate manifested a weight gain of at least 15 pounds compared to 2% in patients who received placebo. In response to these robust findings, numerous other trials have shown that megestrol acetate does indeed improve appetite for patients with advanced cancer. A recent meta-analysis showed that approximately a quarter of patients taking the drug will experienced improved appetite and one in 12 will improve their weight (34). The mean difference for weight gain is approximately 2 kg. Another interesting observation from this analysis is that rates of nausea and vomiting are also improved by megestrol acetate (relative risk =0.58; 95% CI, 0.45–0.74). Although previous studies have shown a direct dose response effect—the higher the dose, the greater the improvement in appetite—a plateau effect does occur, and, for this reason, the recommended dose is 800 mg per day. With more bioavailable formulations the appropriate dose might be lower. As further relevant to megestrol acetate, the most noteworthy potential serious adverse event is thrombophlebitis (34). Indeed, we contend that the risks of treating a patient who has had a previous episode of thrombophlebitis far outweigh the benefits of appetite stimulation. Another potential risk is adrenal insufficiency with abrupt drug withdrawal. Further, male patients have reported impotence and female patients have reported menstrual bleeding with drug withdrawal. Patients should be informed of this adverse event profile prior to the initiation of therapy.
Corticosteroids
Corticosteroids are among the earliest medications used in symptom palliation. Their benefits in palliating cancer-associated loss of appetite were first demonstrated at the Mayo Clinic in the early 1970s (35). In the first randomized, double-blinded, controlled trial for treating cancer-associated anorexia, patients with advanced gastrointestinal malignancy deemed unsuitable for systemic chemotherapy were randomized to escalating doses of dexamethasone versus placebo. At 4 weeks, appetite was significantly improved in the patients receiving steroids, but there was no discernible difference in weight gain or performance ability which led the authors to conclude the observed improvement is largely a result of “euphoric effect” of steroids. Spurred by these results, numerous other studies evaluating corticosteroids for their orexigenic effects have since been completed. The results were similar and favorable in showing that corticosteroids boost appetite. The dose can be small and in the range of dexamethasone 1 milligram twice per day or even less. Short-term steroid usage improves appetite and, by some studies, it also improves sense of wellbeing in patients with advanced cancer (36-38). At least one study has compared dexamethasone and megestrol acetate head-to-head. Though both drugs were similar with regard to efficacy for appetite enhancement, dexamethasone had higher discontinuation rates from toxicity (39). The health risks of long-term steroid usage are well recognized and include metabolic changes, higher fracture rates, cataracts, gastrointestinal discomfort, and changes in mood or behavior. Weighing the risk and benefits of steroid use in patients with cancer, their use is best reserved for patients who have a short life expectancy in the range of only weeks. If a patient has a longer life expectancy and has no contraindications to megestrol acetate, the latter is the preferred agent for appetite stimulation.
Anamorelin
Anamorelin is an oral ghrelin mimetic that was tested in 800+ patients who participated in concurrent phase III trials (40,41). Although it was hoped that this agent would be of great value in not only boosting appetite but also augmenting functional lean tissue, the latter was not the case. However, anamorelin did demonstrate its ability to palliative anorexia in lung cancer patients with advanced disease. This agent is not yet clinically available, but these results should be noted as this agent continues to be developed for clinical use.
Cannabinoids
In popular media, many advocate for the use of cannabis in the treatment of cancer-related symptoms. The strongest evidence backing the use of cannabinoids in supportive care is for treating of chemotherapy-induced nausea, but it has been studied for numerous other applications including analgesia, muscle relaxation, mood/anxiety, insomnia, and appetite (42). In a small phase II study of delta-9-tetrahydrocannabinol (THC), 13 out of 18 patients with cancer-related anorexia reported improvement in their appetite during the course of a 28 day study (43). Interest in the drug for appetite purposes grew, and a phase III study was conducted by the Cannabis In Cachexia Study Group (44). In this trial, 289 cachectic patients were randomized to receive cannabis extract, THC, or placebo, and high rates of appetite improvement were observed in all groups (73%, 58%, and 69%, respectively) with comparison showing no significant difference among the interventions. Quality of life was also measured and unaffected by the cannabis derivatives. In comparison to megestrol acetate, THC (Dronabinol) is inferior for appetite improvement and weight gain (45). Further, it adds no benefit when used in combination with megestrol acetate. Importantly, little research has been undertaken with marijuana itself which has a more complex chemical composition. Previous studies in relatively healthy individuals suggest marijuana does have orexigenic effects. For now, although marijuana derivatives do not appear to have a strong indication for appetite stimulation in advanced cancer patients, clearly more research is needed in the study of marijuana itself.
Other appetite stimulants
Appetite is an expression of complex neuroendocrine and neuronal physiology involving the peripheral nervous system and the brain. The serotonergic system has been implicated with abnormally high levels of plasma and CNS tryptophan, serotonin’s precursor, being observed in patients with cancer anorexia (46). Efforts at leveraging serotonin’s known effects on appetite have yielded only mixed results. As a first example, mirtazapine is a widely used antidepressant known to antagonize post-synaptic 5-HT2 and 5-HT3 receptors. A pilot study assessed the effect of the drug on numerous cancer related symptoms, and though not statistically significant, mirtazapine did result in a trend toward improved appetite at 7 weeks (47). It also resulted in improved weight and mood symptoms. To our knowledge, no large controlled trials have evaluated the effect of mirtazapine in patients with cancer-associated anorexia and weight loss.
Another centrally-acting agent evaluated in cancer-associated anorexia is olanzapine, which is an atypical antipsychotic that acts through multiple neurotransmitters including serotonin and dopamine. Its use is commonly associated with weight gain in other populations, and it has the added benefit of being a potent antiemetic (48). Used in combination with megestrol acetate, olanzapine resulted in greater improvement in weight and appetite compared to megestrol acetate alone (49). Though promising, these results are from a single institution study and have yet to be replicated.
Cyproheptadine is a first generation antihistamine that is also a serotonergic antagonist known to be associated with weight gain in both healthy and unhealthy populations (50,51). Unfortunately, a controlled trial of the drug in weight-losing cancer patients did not result in amelioration of weight loss (52). One tumor-specific situation where patients may benefit from cyproheptadine is metastatic carcinoid tumors with associated loss of appetite (53). Although the previous trial was conducted with goal of demonstrating that cyproheptadine conferred antineoplastic effects, these investigators observed weight gain in a significant portion of the patients. This weight gain was thought to be mediated by peripheral rather than central action of the drug and was interpreted as evidence of appetite stimulation.
Conclusions
In summary, loss of appetite is a distressing symptom that occurs in the majority of patients with advanced cancer. Healthcare providers should be aware of this symptom, its implications, and its challenges from the standpoint of quality of life and survival. Though there are some palliative treatments available for stimulating appetite, their ability to improve quality of life and survival is limited. As some of these agents confer a small but real potential for harm, the role of appetite stimulants should be clearly defined and explained to patients prior to prescribing them. Overall, the sobering data presented here serve as a call to healthcare providers for further clinical trials to evaluate novel approaches for stimulating appetite in weight-losing patients with advanced cancer.
Acknowledgements
Funding: This work was supported by R01CA195473 from the United States’ National Cancer Institute.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget 2017;8:79884-96. [Crossref] [PubMed]
- Doherty M, Khan F, Biswas FN, et al. Symptom Prevalence in Patients with Advanced, Incurable Illness in Bangladesh. Indian J Palliat Care 2017;23:413-8. [Crossref] [PubMed]
- Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 2000;8:175-9. [Crossref] [PubMed]
- Lis CG, Gupta D, Grutsch JF. Can anorexia predict patient satisfaction with quality of life in advanced cancer? Supportive Care in Cancer 2009;17:129-35. [Crossref] [PubMed]
- Reid J, McKenna HP, Fitzsimons D, et al. An exploration of the experience of cancer cachexia: what patients and their families want from healthcare professionals. Eur J Cancer Care (Engl) 2010;19:682-9. [Crossref] [PubMed]
- Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncology 2009;10:865-71. [Crossref] [PubMed]
- Koretz RL, Lipman TO, Klein S, et al. AGA technical review on parenteral nutrition. Gastroenterology 2001;121:970-1001. [Crossref] [PubMed]
- August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472-500. [Crossref] [PubMed]
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. [Crossref] [PubMed]
- Dans M, Smith T, Back A, et al. NCCN Guidelines Insights: Palliative Care, Version 2.2017. J Natl Compr Canc Netw 2017;15:989-97. [Crossref] [PubMed]
- Panton LB, Rathmacher JA, Baier S, et al. Nutritional supplementation of the leucine metabolite beta-hydroxy-beta-methylbutyrate (hmb) during resistance training. Nutrition 2000;16:734-9. [Crossref] [PubMed]
- Barbul A, Lazarou SA, Efron DT, et al. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 1990;108:331-6; discussion 6-7. [PubMed]
- Clark RH, Feleke G, Din M, et al. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter Enteral Nutr 2000;24:133-9. [Crossref] [PubMed]
- May PE, Barber A, D'Olimpio JT, et al. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg 2002;183:471-9. [Crossref] [PubMed]
- Deutz NE, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr 2011;30:759-68. [Crossref] [PubMed]
- Berk L, James J, Schwartz A, et al. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer 2008;16:1179-88. [Crossref] [PubMed]
- Liu S, Wu HJ, Zhang ZQ, et al. L-carnitine ameliorates cancer cachexia in mice by regulating the expression and activity of carnitine palmityl transferase. Cancer Biol Ther 2011;12:125-30. [Crossref] [PubMed]
- Kraft M, Kraft K, Gartner S, et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)--a randomized multicentre trial. Nutr J 2012;11:52. [Crossref] [PubMed]
- Lanhers C, Pereira B, Naughton G, et al. Creatine Supplementation and Upper Limb Strength Performance: A Systematic Review and Meta-Analysis. Sports Medicine 2017;47:163-73. [Crossref] [PubMed]
- Jatoi A, Steen PD, Atherton PJ, et al. A double-blind, placebo-controlled randomized trial of creatine for the cancer anorexia/weight loss syndrome (N02C4): an Alliance trial. Ann Oncol 2017;28:1957-63. [Crossref] [PubMed]
- Nabavi SF, Bilotto S, Russo GL, et al. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev 2015;34:359-80. [Crossref] [PubMed]
- Whitehouse AS, Smith HJ, Drake JL, et al. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001;61:3604-9. [PubMed]
- Fearon KC, Von Meyenfeldt MF, Moses AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 2003;52:1479-86. [Crossref] [PubMed]
- Bruera E, Strasser F, Palmer JL, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: A double-blind, placebo-controlled study. J Clin Oncol 2003;21:129-34. [Crossref] [PubMed]
- Jatoi A, Rowland K, Loprinzi CL, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: A North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 2004;22:2469-76. [Crossref] [PubMed]
- Dewey A, Baughan C, Dean T, et al. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev 2007.CD004597. [PubMed]
- Ovesen L, Allingstrup L, Hannibal J, et al. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. J Clin Oncol 1993;11:2043-9. [Crossref] [PubMed]
- Baldwin C, Spiro A, McGough C, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet 2011;24:431-40. [Crossref] [PubMed]
- Persson CR, Johansson BB, Sjoden PO, et al. A randomized study of nutritional support in patients with colorectal and gastric cancer. Nutr Cancer 2002;42:48-58. [Crossref] [PubMed]
- Baldwin C, Spiro A, Ahern R, et al. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:371-85. [Crossref] [PubMed]
- Isenring E, Capra S, Bauer J. Patient satisfaction is rated higher by radiation oncology outpatients receiving nutrition intervention compared with usual care. J Hum Nutr Diet 2004;17:145-52. [Crossref] [PubMed]
- Oberholzer R, Hopkinson JB, Baumann K, et al. Psychosocial effects of cancer cachexia: a systematic literature search and qualitative analysis. J Pain Symptom Manage 2013;46:77-95. [Crossref] [PubMed]
- Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990;82:1127-32. [Crossref] [PubMed]
- Ruiz Garcia V, Lopez-Briz E, Carbonell Sanchis R, et al. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 2013.CD004310. [PubMed]
- Moertel CG, Schutt AJ, Reitemeier RJ, et al. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer 1974;33:1607-9. [Crossref] [PubMed]
- Della Cuna GR, Pellegrini A, Piazzi M. Effect of methylprednisolone sodium succinate on quality of life in preterminal cancer patients: a placebo-controlled, multicenter study. The Methylprednisolone Preterminal Cancer Study Group. Eur J Cancer Clin Oncol 1989;25:1817-21. [Crossref] [PubMed]
- Willox JC, Corr J, Shaw J, et al. Prednisolone as an appetite stimulant in patients with cancer. Br Med J (Clin Res Ed) 1984;288:27. [Crossref] [PubMed]
- Popiela T, Lucchi R, Giongo F. Methylprednisolone as palliative therapy for female terminal cancer patients. The Methylprednisolone Female Preterminal Cancer Study Group. Eur J Cancer Clin Oncol 1989;25:1823-9. [Crossref] [PubMed]
- Loprinzi CL, Kugler JW, Sloan JA, et al. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol 1999;17:3299-306. [Crossref] [PubMed]
- Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016;17:519-31. [Crossref] [PubMed]
- Currow D, Temel JS, Abernethy A, et al. ROMANA 3: a phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann Oncol 2017;28:1949-56. [Crossref] [PubMed]
- Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer 2003;11:137-43. [PubMed]
- Nelson K, Walsh D, Deeter P, et al. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J Palliat Care 1994;10:14-8. [PubMed]
- Cannabis In Cachexia Study G, Strasser F, Luftner D, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 2006;24:3394-400. [Crossref] [PubMed]
- Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 2002;20:567-73. [Crossref] [PubMed]
- Cangiano C, Cascino A, Ceci F, et al. Plasma and CSF tryptophan in cancer anorexia. J Neural Transm Gen Sect 1990;81:225-33. [Crossref] [PubMed]
- Theobald DE, Kirsh KL, Holtsclaw E, et al. An open-label, crossover trial of mirtazapine (15 and 30 mg) in cancer patients with pain and other distressing symptoms. J Pain Symptom Manage 2002;23:442-7. [Crossref] [PubMed]
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2016;375:134-42. [Crossref] [PubMed]
- Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: a randomized trial. Support Care Cancer 2010;18:951-6. [Crossref] [PubMed]
- Noble RE. Effect of cyproheptadine on appetite and weight gain in adults. JAMA 1969;209:2054-5. [Crossref] [PubMed]
- Rahman KM. Appetite stimulation and weight gain with cyproheptadine (periactin) in tuberculosis patients (double-blind clinical study). Med J Malaysia 1975;29:270-4. [PubMed]
- Kardinal CG, Loprinzi CL, Schaid DJ, et al. A controlled trial of cyproheptadine in cancer patients with anorexia and/or cachexia. Cancer 1990;65:2657-62. [Crossref] [PubMed]
- Moertel CG, Kvols LK, Rubin J. A study of cyproheptadine in the treatment of metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer 1991;67:33-6. [Crossref] [PubMed]