Usage of EGFR-TKI and WBRT in NSCLC patients with brain metastases
Central nervous system (CNS) metastases, including brain metastases (BM) and leptomeningeal metastases (LM), represent a common manifestation in patients with non-small-cell lung cancer (NSCLC). About 10% to 20% of NSCLC patients have BM and/or LM in their initial diagnosis of lung cancer. Approximately 20% to 30% of the remaining NSCLC patients will eventually have CNS metastases at some point during the course of the disease (1-5). The appearance of CNS metastases is often accompanied by disabling neurological symptoms, a decrease in quality of life, and a dismal outlook for survival; the patients usually died of CNS failure before other organ failure (2,3). As such, an optimization of the treatment algorithms for NSCLC-related CNS metastases would be of major relevance for these patients.
In the last decade, the introduction of tyrosine kinase inhibitors (TKI), such as erlotinib and gefitinib, targeting the epidermal growth factor receptor (EGFR), has resulted in a major therapeutic improvement for NSCLC patients with activating EGFR mutations (6,7). Recent clinical studies report cerebral response rates of 75-89% in EGFR-mutated NSCLC patients who had cerebral metastases and were treated with EGFR-TKI alone, or 84% when combining EGFR-TKI with whole brain radiotherapy (WBRT) (4). This response rate was considerably higher than what would be expected for standard approaches like WBRT with or without systemic chemotherapy. Usage of EGFR-TKI alone without WBRT in EGFR-unselected NSCLC patients yielded a cerebral response rate ranging from 10% to 70%, and 71% to 81% in studies combining EGFR-TKI with WBRT (4). Thus, it is not therapeutically appropriate to use EGFR-TKI alone, without brain irradiation, in tumor EGFR-unselected patients. However, it is emerging opinion and suggested that EGFR-mutated NSCLC patients with cerebral metastases could receive EGFR-TKI first, and then delay brain irradiation until either CNS imaging or symptom progression, in order to delay WBRT-related neurological sequels (8,9).
Welsh et al., reported on a prospective study entitled “Phase II Trial of Erlotinib Plus Concurrent Whole-Brain Radiation Therapy for Patients With Brain Metastases From Non-Small-Cell Lung Cancer” in a recent issue of the “Journal of Clinical Oncology” (10). They proposed that activation of EGFR contributes to radiation resistance and that use of EGFR-TKI could overcome or reduce this resistance. In this trial, 40 EGFR-unselected patients received one week of erlotinib treatment, followed by concurrent WBRT, then erlotinib maintenance therapy. The number of patients with newly diagnosed NSCLC was not reported in the article. NSCLC patients with brain metastasis newly diagnosed radiographically, with or without prior craniotomy or stereotactic radiosurgery, could be enrolled into the study. Seventeen patients had tumor EGFR analyzed. Eight of these 17 EGFR mutation status-known patients received prior systemic chemotherapy. The number of patients that received prior craniotomy or stereotactic radiosurgery was not reported in the paper. Among the 40 patients treated, the number of brain metastases was 0-3 in 18 patients, 4-10 in 15 patients, and >10 in 7 patients. Since nearly half of the study population had only 3 or fewer brain metastases and more than 80% of the patients had brain metastases numbering 10 or less, these patients would also be potential candidates for neurosurgery or stereotactic radiosurgery (such as γ-knife radiotherapy) in today’s clinical practice (11). WBRT leads to more neurocognitive problems than radiosurgery, and this may occur especially in patients with an EGFR mutation who had a longer survival than the EGFR wild-type patients.
The authors reported that no statistically significant differences were found between the erlotinib group and the historical control group in the proportion of patients with evidence of neurotoxicity at any of the time points examined, regardless of whether the comparison was related to change from baseline or change from the previous test score. This means there was no augmentation of neurotoxicities by erlotinib treatment when receiving WBRT. However, there was no WBRT-alone arm in this trial, with the result that readers could not evaluate the benefit of the addition of erlotinib to WBRT treatment, even though there was no augmentation of toxicities compared to the historical control.
In the study, the authors concluded that “The higher-than-expected rate of EGFR mutations in these unselected patients raises the possibility that EGFR-mutated tumors are prone to brain dissemination”. This conclusion should be more conservative, because not all patients were newly diagnosed NSCLC patients with brain metastases at initial staging, and more patients with EGFR activating mutation live long enough to develop CNS metastases compared to EGFR wild-type patients. Eichler et al., reported 93 NSCLC patients with a known EGFR mutation status that initially presented or developed brain metastases during the disease course (12). They found that 20 of 41 patients (48.8%) with EGFR mutation, and 24 of 52 EGFR wild-type patients (46.2%), respectively, had brain metastases at the time of initial lung cancer diagnosis. Thus, the relatively high proportion of EGFR-mutated patients among the patients with brain metastases is likely due to treatment selection, while the incidence was similar in the initial lung cancer diagnosis.
We need to interpret carefully the data regarding EGFR mutation status and its relationship to treatment efficacy, because only 17 of 40 patients had EGFR mutation status data, with the result that we could not conclude EGFR inhibition could reverse radiation resistance in either EGFR wild-type or mutated patients. Readers will be more interested in whether or not EGFR inhibition could reverse radiotherapy-resistance in EGFR wild-type patients who received WBRT, because EGFR-mutated patients responded to EGFR-TKI treatment well, including their CNS lesions. Reaching a conclusion from this study as to whether EGFR inhibition could reverse radiation resistance in either EGFR wild-type or mutated patients would be difficult. It would be better if this study were performed only on EGFR wild-type patients. Nevertheless, this study provides physicians another option of treatment for these patients that is relatively safe and well tolerated.
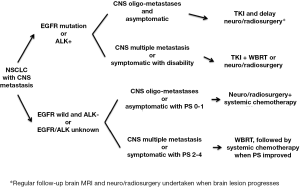
In conclusion, it is suggested that NSCLC patients should receive tumor EGFR mutation analysis, followed by EGFR-TKI treatment, and that a delay in undertaking neurosurgery, stereotactic radiosurgery, or WBRT in patients without obvious neurological signs or symptoms should be considered. For patients with EGFR wild-type, an ALK test should be done when available and ALK-TKI be given. For EGFR wild-type patients, combining neurosurgery, stereotactic radiosurgery, or WBRT with systemic chemotherapy is the standard of treatment (Figure 1).
Acknowledgements
Disclosure: The author declares no potential conflict of interest.
References
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [PubMed]
- Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol 2013;8:185-91. [PubMed]
- Wu WS, Chen YM, Tsai CM, et al. Changing causes of death post-epidermal growth factor receptor-tyrosine kinase inhibitor era in patients with advanced non-small-cell lung cancer. J Clin Oncol 2012;30:abstr e18132
- Berger LA, Riesenberg H, Bokemeyer C, et al. CNS metastases in non-small-cell lung cancer: current role of EGFR-TKI therapy and future perspectives. Lung Cancer 2013; Available online: http://dx.doi.org/ [PubMed]
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193-9. [PubMed]
- Hirsch FR, Jänne PA, Eberhardt WE, et al. Epidermal growth factor receptor inhibition in lung cancer: status 2012. J Thorac Oncol 2013;8:373-84. [PubMed]
- Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line—is there a difference? J Clin Oncol 2013;31:1081-8. [PubMed]
- Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009;65:351-4. [PubMed]
- Olson JJ, Paleologos NA, Gaspar LE, et al. The role of emerging and investigational therapies for metastatic brain tumors: a systematic review and evidence-based clinical practice guideline of selected topics. J Neurooncol 2010;96:115-42. [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. Available online: http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2011.40.1174
- Non-small cell lung cancer. National Comprehensive Cancer Network guidelines version 2.2013. Available online: http//www.nccn.com
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193-9. [PubMed]


