Bleeding in cancer patients and its treatment: a review
Scope of the problemOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
Bleeding is a frequent problem for patients with advanced cancer, with approximately 10% of all patients having at least one episode and almost 30% in patients with hematologic malignancies (1). These episodes may range from low-grade oozing to major episodic bleeding or even catastrophic bleeds. Bleeding can be caused by the cancer itself, as with local tumor invasion, abnormal tumor vasculature, or tumor regression. It may also be related to the anti-tumor treatments including prior radiation therapy or chemotherapy. It can be exacerbated by immunotherapies such as bevacizumab, nonsteroidal anti-inflammatories drugs (NSAIDs), and anticoagulants that are routinely used in cancer patients. Patients may also be predisposed to bleeding due to thrombocytopenia from the malignancy or induced by chemotherapy.
There is limited literature studying palliative treatments for hemostasis in the context of advanced cancer, and no randomized therapeutic trials. Randomized trials are difficult in this setting, given the complex patient population, the variety of sites affected by bleeding, and the variety of treatment modalities involving multiple medical specialties. Most studies examine a single modality, and there are no consistent definitions of bleeding or treatment response. Existing literature is also inconsistent in outcome measures, time points, and method assessment. Much of the literature is retrospective, so there is inherent difficulty in standardizing endpoint definition and evaluation.
Goals of care and communicationOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
Goals of care should be discussed as an integral part of considering therapies in patients at high risk of bleeding or suffering from its effects. Patients may find bleeding visible and disturbing, or it may have significant effects on their quality of life. It is also important to consider the patient’s estimated life expectancy, which may involve the use of prognostic models (2-8). The rapidity of control of bleeding should be considered, but so too should the comfort of the patient during the treatment process. For example, radiation therapy can usually control bleeding within 24–48 hours, but patients have to be comfortable lying on the treatment table for the planning and treatment process. Surgery too can help manage bleeding tumors, but the burden and duration of recovery should be considered in the context of the patient’s life expectancy and goals of care.
For patients who suffer a major episode of bleeding but are not at the end of life, establishment of intravenous access, stabilization with fluids, and hemodynamic monitoring may allow investigation into the cause of bleeding. Analysis should include a complete blood count, coagulation profile, and a complete metabolic panel with assessment of liver enzymes and function. It may be useful to perform imaging studies including computed tomography or angiography of the area suspected of bleeding, and/or endoscopy. Possible contributing factors including comorbidities, medications, and recent therapeutic interventions should be examined. In particular, if the patient is on anticoagulation therapy, the risks of further bleeding versus those of clotting should be examined and discussed. Use of oral anticoagulants has been associated with genitourinary cancer in atrial fibrillation patients with hematuria, so it is important to consider stopping it, and to carefully evaluate these patients for the cause of hematuria (9).
For patients at risk of catastrophic bleeding, patients and their families should be prepared for the visually and mentally disturbing effects of such an episode. Encourage the use of dark sheets, towels, blankets, and clothing to reduce the visual shock of seeing a massive bleed. Fast acting sedatives such as intravenous or subcutaneous midazolam should be available, and families should be instructed on their use if the patient is at home. Although terminal sedation may be appropriate for bleeding at the end of life, a catastrophic bleed may cause death rapidly and there may not be time for sedation.
Discontinuation of causative/exacerbating agentsOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
One of the most critical components of the assessment of patients with bleeding is a thorough assessment of potential causative or exacerbating agents. Obviously, the medications that fall into this category cannot be fully discussed in this manuscript, but the most common medications for advanced cancer patients include NSAIDs and anticoagulants. Anti-inflammatories are often used to treat pain for patients with advanced cancer, but it is important to consider their anti-platelet and anti-coagulant properties that may exacerbate bleeding. Similarly, patients with advanced cancer are often on anticoagulants such as warfarin or enoxaparin, which necessitates the considerations of the risks of further bleeding against the risks of deep venous or pulmonary thromboembolism. Among patients on anticoagulation, patients with cancer develop bleeding complications at a higher rate than those without cancer (10). INR control is difficult in the setting of cancer treatments, so use of oral anticoagulation during the first year after cancer diagnosis can increase bleeding and other major cardiac adverse events (11).
The effect of chemotherapy agents and radiation therapy on thrombocytopenia should also be considered, as this may increase the risk of bleeding. If considered a critical contributor, these agents may be held to allow bone marrow recovery and resolution of thrombocytopenia.
Systemic therapiesOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
Transfusions of whole blood or blood products can be given to resuscitate patients who are hemodynamically unstable and actively bleeding. The AABB (formerly the American Association of Blood Banks) has evidence-based guidelines for the transfusion of red blood cells, platelets, and plasma (12-14). It is less clear how to use transfusions for the palliative treatment of patients with advanced malignancy, although symptomatic improvement has been seen in these patients (15).
Vitamin K can be used to correct coagulation for patients on warfarin or those with deficiencies of the vitamin K-dependent clotting factors (factors II, VII, IX, X). Vitamin K can be given orally, subcutaneously, or intravenously.
Tranexamic acid has not been studied in advanced cancer, but it reduces mortality due to bleeding by approximately one-third. A reduction of approximately one-third in blood loss and transfusion requirements has been seen in meta analyses of its use in elective surgery as well (16,17). Tranexamic acid is currently being evaluated in gastrointestinal bleeding. There have been minimal side effects associated with its administration, and no studies have shown an increased thrombotic risk. There is an increased risk of neurologic complications with increasing doses of tranexamic acid. No dose-response has been seen for its therapeutic effect, and the recommended dose is 10 mg/kg per dose given intravenously every 6–8 hours, with no benefit to doses above 1 gram (18).
Local therapiesOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
Dressings, packing, and topical agents
Patients with bleeding skin lesions can have non-adherent dressings applied, and those with bleeding at accessible skin or mucosal sites can be treated with topical agents including absorbable gelatin or collagen. Nasal, vaginal, or rectal bleeding can be limited with packing. Vaginal bleeding can also be treated with topical application of Moh’s paste and Monsel’s solution (19).
Radiation therapy
Radiation therapy has been shown to decrease gastrointestinal bleeding (20). hemoptysis (21-31), hematuria (32-34), and vaginal bleeding (35). If patients are hemodynamically stable enough for transport to the radiation department, radiation therapy to palliate bleeding can be delivered in a small number of treatments and can be effective within 24–48 hours of the delivery of the first dose. The variety of treatment regimens for palliation of bleeding including single treatments of 8–10 Gray (Gy), intermediate courses of 4–8 Gy given in 3–5 treatments, or longer courses of 30–45 Gy in 10–15 treatments. No treatment scheme has been proved to be more effective than another when used for palliation of bleeding, but at least one randomized trial suggests that side effects are less likely with shorter treatment courses (36).
Patients with advanced and metastatic cancers may have equal or better palliation with shorter radiation courses, with improved convenience and reduced costs (37). Another set of patients who may benefit from palliative radiation therapy are those who would have curable cancers but are too medically frail for intense curative treatment. These patients may have a longer life expectancy than those with advanced cancer, putting them at higher risk for potential late complications of radiation therapy months to years after treatment. Thus it may be less appropriate to use some of the treatment schemes with the highest dose per fraction, as these have increased risk of late complications. Another important consideration is if the patient has a history of prior radiation to the same anatomic site. Re-irradiation may be an option if the benefits outweigh the risks, but care must be taken to respect the constraints of critical normal tissues, especially the spinal cord.
Endoscopic procedures
Endoscopic procedures including bronchoscopy, esophagogastroduodenoscopy, cystoscopy, and colonoscopy, can all be used to identify and treat bleeding tumors in the visualized organs. Treatment options have been described using cautery, argon plasma coagulation, clip deployment, injections of epinephrine or other sclerosing agents, or laser therapy. Rates of success and re-bleed vary, but endoscopic treatments are most likely to be successful in the setting of less-advanced tumors and those without diffuse bleeding. Of note, two small series of the use of hemostatic powder on a bleeding tumor reported hemostasis in 100% of patients, but re-bleeding remained a problem (38,39). Similarly, argon plasma coagulation, a non-contact thermal cautery that penetrates 2–3 mm, showed immediate hemostasis in 100% of patients, but had a 30% rate of re-bleeding (40).
Transcutaneous embolization
Transcutaneous embolization can be used with a variety of mechanical devices (41) or sclerosing agents. Mechanical devices such as coils are defined by the size of their cores, diameters, and lengths. Sclerosing agents can be biodegradable or permanent, depending on the indication; permanent agents such as polyvinyl alcohol or microspheres are generally used for malignant bleeding (42). Appropriate patients must be able to lie flat throughout the procedure, with an identified bleeding vessel that can be catheterized and selectively embolized. Vessels supplying the normal tissues must be protected while the blood supply of the tumor is embolized. Any pre-existing coagulopathies must be corrected and the patient must be well-hydrated as contrast agents are used to visualize vasculature. Within these parameters, successful hemostasis is reached in 70–99% of patients (43), but re-bleeding can occur. Early re-bleeds are usually due to incomplete embolization, whereas late re-bleeding is usually due to recanalization of the vessels. Complications include bruising or hematoma of the local site, bleeding, coil migration, vessel occlusion, or post-embolization syndrome. Necrosis of the tumor may follow embolization and cause up to several days of pain, flu-like symptoms, or nausea and vomiting (42,43).
Surgery
Surgical procedures to relieve bleeding may include vessel ligation or resection of a bleeding tumor and/or organ. They may relieve bleeding as warranted by amount of bleeding, life expectancy, and lack of other treatment options. It is also important to consider the anesthesia risk. Laparoscopic procedures may cause less acute morbidity than open procedures but may have a higher cost.
Treatment for selected sites of hemorrhageOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
Skin lesions
- Case 1: A 43-year-old patient with squamous cell carcinoma of an unknown primary of head and neck, presenting with bleeding wound. Prior to treatment he had a large bleeding chin wound (Figure 1A). Following radiation treatment to 20 Gy delivered in 2 weekly fractions, his tumor burden had greatly decreased and his wound was no longer bleeding (Figure 1B).
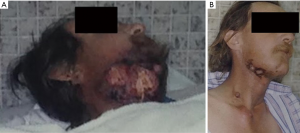
Skin lesions from metastatic disease can ooze, bleed, smell foul, or be painful. Local management options for skin lesions include the use of non-adherent dressings, surgical excision, radiation therapy, or other ablative therapy. Superficial lesions may be treated sufficiently with laser or cryotherapy. Palliative radiation therapy to manage symptoms of pain and bleeding can be treated with a short, hypofractionated regimen such as 20 Gy delivered in 5 daily fractions or 20 Gy in 2 weekly fractions.
Electrochemotherapy combines a cytotoxic drug with electrical impulses to increase the cell membrane’s permeability, which enhances uptake of the drug. One example, with bleomycin as the cytotoxic drug, has been shown to have response rates of 77–87%. The electrical impulses involved in this treatment generally cause painful muscle contractions, so local or general anesthesia is generally required (44).
Injection of interleukin-2 into a tumor can be delivered 2–3 sessions per week in doses of 3–18 MIU per session. Response rates of 70–80% have been reported. Melanoma or sarcoma can be treated with isolated limb perfusion (44).
Hemoptysis
- Case 2: A 55-year-old patient with non-small cell lung cancer presenting with hemoptysis. Prior to treatment, he was having significant, frequent hemoptysis and was intubated. He was treated with 17 Gy in 2 weekly fractions (Figure 2A,B) and hemoptysis resolved. He was extubated, regained strength, and started chemotherapy. He died 4 months later without recurrence of bleeding.
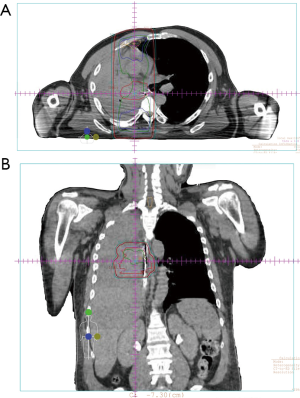
Patients with large volume hemoptysis may require intubation for airway protection, based on the patient’s wishes. Treatment may then proceed by bronchoscopic interventions, angiography and embolization, or radiation therapy. Of note, single lumen tubes will pass a standard flexible bronchoscope but they do not permit reliable lung isolation. Rigid bronchoscopy is more useful for rapid suctioning of large volume bleeding, but it is challenging, requires expertise, and is best performed in the operating room.
Bronchoscopy may allow visualization and a variety of treatment interventions. Visualization of airways and bleeding sources may be possible with suctioning of bleeding and clots. Balloon catheters via the scope can help provide temporary control of bleeding. Options for interventions may include balloon tamponade, iced saline lavage, Nd-YAG laser coagulation, electrocautery, or argon plasma coagulation. Hemostasis has been reported in 60% (for Nd-YAG laser) to 100% (for argon plasma coagulation) (45).
Bronchial artery angiography and embolization may be appropriate for lesions that are not amenable to bronchoscopy. Of note, it is particularly critical to avoid the spinal artery during embolization, as it risks spinal cord injury (46). Hemoptysis on angiography may demonstrate tumor blush or active extravasation. Hemostasis rates of embolization in malignancy are difficult to determine, because most studies are not limited to cancer patients.
Radiation therapy results in hemostasis in 80–97% of hemoptysis patients (21,27). Fractionation regimens have included 17 Gy in 2 weekly fractions (8.5 Gy/fraction) (28,30), 20 Gy in 5 daily fractions (4 Gy/fraction), and 30–39 Gy in 10–13 fractions (3 Gy/fraction) (25,26). No consistent differences in rates of palliation have been reported. Studies have also demonstrated conflicting results for survival, although studies were not powered to detect a survival benefit and included curable patients. Radiation myelitis has been reported only rarely as a complication in patients surviving at least 9 months post-radiation (47). The increased convenience and reduced cost of shorter courses thus favors their use over longer courses of radiation (21,28,30). Three-dimensional conformal radiation therapy techniques can also be used to decrease the spinal cord dose and limit the small risk of radiation myelitis.
Vaginal bleeding
Vaginal bleeding commonly occurs in advanced gynecological cancer including cervical and endometrial cancer. Topical therapies include application of Moh’s paste or Monsel’s solution to areas of vaginal bleeding, or vaginal packing which may soaked with paraformaldehyde. Interventional radiology services can perform uterine or iliac artery embolization, using mechanical devices such as coils or sclerosing agents. A more invasive treatment option can be surgical ligation of vessels if interventional radiology services are not available (19).
Palliative radiation therapy can also be directed at the uterus and/or cervix. Large 10-Gy, monthly radiation fractions with misonidazole have been investigated for palliation of advanced pelvic malignancies. Although approximately 40% of patients achieved complete or partial response, the study was a small stage I/II trial and the rate of gastrointestinal complications was considered unacceptably high (48). However, many other fractionation schemes are effective for palliation of vaginal bleeding, including 21 Gy in 3 weekly fractions (7 Gy/fraction), 20 Gy in 5 daily fractions (4 Gy/fraction), or 14.8 Gy in 4 twice-daily fractions (3.7 Gy/fraction). For patients with potentially curable disease who may live long enough to be at risk for late complications of radiation therapy, care should be taken to mitigate the risks related to large fraction sizes.
Gastrointestinal bleeding
- Case 3: A 90-year-old patient with localized rectal cancer s/p rectal stent presenting with large-volume rectal bleeding, unable to undergo surgical resection due to severe aortic stenosis. He was treated with 20 Gy in four daily fractions (Figure 3). His bleeding stopped within days, before the treatment course was completed. He died without recurrence of his cancer.
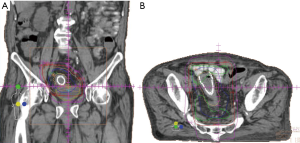
Palliative radiation therapy has been used to treat bleeding from a variety of gastrointestinal tumors, with a variety of regimens. Although there is relatively sparse outcomes data, hemostasis has been reported in 50–73% of patients with locally advanced gastric cancer treated with radiation (49). This wide range of outcomes may be due to variations in the definition of hemostasis, with include no further bleeding, decreased transfusion requirements, or an increase in hemoglobin levels. A recent systematic review of 27 studies on the use of radiation therapy to treat bleeding in rectal cancer patients reported 81% of patients achieving hemostasis (50).
Hematuria
Hematuria is often due to tumor invasion of the bladder, so initial therapies may include bladder irrigation and discontinuing medications that increase bleeding risk such as anti-inflammatories or anticoagulants. Surgical options may include transurethral resection of the bladder with coagulation, or cystectomy with urinary diversion. Radiation therapy with various regimens have been used and report 50–92% hemostasis, with a range of 3–8 Gy/fraction (51). Patients with bleeding from bladder tumors may also achieve hemostasis by embolization of branches of the anterior trunk of the iliac artery (44). Renal artery embolization can be used to relieve hematuria due to malignant renal tumors, as well as any associated flank pain (34). A historical treatment for hematuria was intravesicular instillation of formalin, but this is no longer used due to discomfort, the risk of renal failure, and a need for general or spinal anesthesia (52). Instead, alum or prostaglandins may be instilled into the bladder, with varying rates of hemostasis. All of these instillation treatments work by causing protein precipitation that occludes the bleeding vessels. Prostaglandin instillation is generally reserved for cases of alum failure, due to the increased cost, and issues of availability and storage (15).
ConclusionsOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
Bleeding is a common complication for patients with advanced cancer, but the approach to prevention and treatment will vary substantially based on the type and site of bleeding. Universal principles include hemodynamic stabilization and care consistent with the patient’s goals of care. Exacerbating agents such as anticoagulants may be discontinued, and blood products may be given as indicated. Accessible sites including the nose, skin, and vagina, may be packed and treated with topical agents. More involved or invasive interventions to treat bleeding include radiation therapy, endoscopic treatments, embolization, and surgery. Unfortunately, literature is limited for the palliative treatment of bleeding in patients with advanced cancer, including few prospective studies, a lack of consistent endpoints, and no randomized trials. Treatment of bleeding patients should proceed based on patient preferences and resource availability.
AcknowledgementsOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
None.
FootnoteOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Scope of the problem
- Goals of care and communication
- Discontinuation of causative/exacerbating agents
- Systemic therapies
- Local therapies
- Treatment for selected sites of hemorrhage
- Conclusions
- Acknowledgements
- Footnote
- References
- Cartoni C, Niscola P, Breccia M, et al. Hemorrhagic complications in patients with advanced hematological malignancies followed at home: An Italian experience. Leuk Lymphoma 2009;50:387-91. [Crossref] [PubMed]
- Chow E, Abdolell M, Panzarella T, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol 2008;26:5863-9. [Crossref] [PubMed]
- Glare P, Sinclair C, Downing M, et al. Predicting survival in patients with advanced disease. Eur J Cancer 2008;44:1146-56. [Crossref] [PubMed]
- Gwilliam B, Keeley V, Todd C, et al. Development of prognosis in palliative care study (PiPS) predictor models to improve prognostication in advanced cancer: Prospective cohort study. BMJ 2011;343:d4920. [Crossref] [PubMed]
- Morita T, Tsunoda J, Inoue S, et al. The Palliative Prognostic Index: A scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer 1999;7:128-33. [Crossref] [PubMed]
- Pirovano M, Maltoni M, Nanni O, et al. A new palliative prognostic score: A first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage 1999;17:231-9. [Crossref] [PubMed]
- Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med 1988;148:1586-91. [Crossref] [PubMed]
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: The TEACHH model. Cancer 2014;120:134-41. [Crossref] [PubMed]
- Yu HT, Kim TH, Uhm JS, et al. Clinical significance of hematuria in atrial fibrillation with oral anticoagulation therapy. Circ J 2017;81:158-64. [Crossref] [PubMed]
- Hutten BA, Prins MH, Gent M, et al. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: A retrospective analysis. J Clin Oncol 2000;18:3078-83. [Crossref] [PubMed]
- Lee YJ, Park JK, Uhm JS, et al. Bleeding risk and major adverse events in patients with cancer on oral anticoagulation therapy. Int J Cardiol 2016;203:372-8. [Crossref] [PubMed]
- Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann Intern Med 2012;157:49-58. [Crossref] [PubMed]
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: A clinical practice guideline from the AABB. Ann Intern Med 2015;162:205-13. [Crossref] [PubMed]
- Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010;50:1227-39. [Crossref] [PubMed]
- Monti M, Castellani L, Berlusconi A, et al. Use of red blood cell transfusions in terminally ill cancer patients admitted to a palliative care unit. J Pain Symptom Manage 1996;12:18-22. [Crossref] [PubMed]
- Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ 2012;344:e3054. [Crossref] [PubMed]
- Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg 2013;100:1271-9. [Crossref] [PubMed]
- Hunt BJ. The current place of tranexamic acid in the management of bleeding. Anaesthesia 2015;70 Suppl 1:50-3, e18.
- Eleje GU, Eke AC, Igberase GO, et al. Palliative interventions for controlling vaginal bleeding in advanced cervical cancer. Cochrane Database Syst Rev 2015;5:CD011000. [PubMed]
- Crane CH, Janjan NA, Abbruzzese JL, et al. Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys 2001;49:107-16. [Crossref] [PubMed]
- Inoperable non-small-cell lung cancer (NSCLC): A Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer 1991;63:265-70. [Crossref] [PubMed]
- A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer 1992;65:934-41. [Crossref] [PubMed]
- Abratt RP, Shepherd LJ, Salton DG. Palliative radiation for stage 3 non-small cell lung cancer--A prospective study of two moderately high dose regimens. Lung Cancer 1995;13:137-43. [Crossref] [PubMed]
- Bezjak A, Dixon P, Brundage M, et al. Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 2002;54:719-28. [Crossref] [PubMed]
- Kramer GW, Wanders SL, Noordijk EM, et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 2005;23:2962-70. [Crossref] [PubMed]
- Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167-75. [Crossref] [PubMed]
- Rees GJ, Devrell CE, Barley VL, et al. Palliative radiotherapy for lung cancer: Two versus five fractions. Clin Oncol (R Coll Radiol) 1997;9:90-5. [Crossref] [PubMed]
- Senkus-Konefka E, Dziadziuszko R, Bednaruk-Mlynski E, et al. A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC). Br J Cancer 2005;92:1038-45. [Crossref] [PubMed]
- Simpson JR, Francis ME, Perez-Tamayo R, et al. Palliative radiotherapy for inoperable carcinoma of the lung: Final report of a RTOG multi-institutional trial. Int J Radiat Oncol Biol Phys 1985;11:751-8. [Crossref] [PubMed]
- Sundstrøm S, Bremnes R, Aasebo U, et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: A national phase III trial. J Clin Oncol 2004;22:801-10. [Crossref] [PubMed]
- Teo P, Tai TH, Choy D, et al. A randomized study on palliative radiation therapy for inoperable non small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 1988;14:867-71. [Crossref] [PubMed]
- Dirix P, Vingerhoedt S, Joniau S, et al. Hypofractionated palliative radiotherapy for bladder cancer. Support Care Cancer. 2016;24:181-6. [Crossref] [PubMed]
- Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: Results of medical research council trial BA09. Int J Radiat Oncol Biol Phys 2000;47:379-88. [Crossref] [PubMed]
- McLaren DB, Morrey D, Mason MD. Hypofractionated radiotherapy for muscle invasive bladder cancer in the elderly. Radiother Oncol 1997;43:171-4. [Crossref] [PubMed]
- Yan J, Milosevic M, Fyles A, et al. A hypofractionated radiotherapy regimen (0-7-21) for advanced gynaecological cancer patients. Clin Oncol (R Coll Radiol) 2011;23:476-81. [Crossref] [PubMed]
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798-804. [Crossref] [PubMed]
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: Cost-utility analysis based on a randomized trial. J Natl Cancer Inst 2003;95:222-9. [Crossref] [PubMed]
- Chen YI, Barkun AN, Soulellis C, et al. Use of the endoscopically applied hemostatic powder TC-325 in cancer-related upper GI hemorrhage: Preliminary experience (with video). Gastrointest Endosc 2012;75:1278-81. [Crossref] [PubMed]
- Leblanc S, Vienne A, Dhooge M, et al. Early experience with a novel hemostatic powder used to treat upper GI bleeding related to malignancies or after therapeutic interventions (with videos). Gastrointest Endosc 2013;78:169-75. [Crossref] [PubMed]
- Thosani N, Rao B, Ghouri Y, et al. Role of argon plasma coagulation in management of bleeding GI tumors: Evaluating outcomes and survival. Turk J Gastroenterol 2014;25 Suppl 1:38-42. [Crossref] [PubMed]
- Delgal A, Cercueil JP, Koutlidis N, et al. Outcome of transcatheter arterial embolization for bladder and prostate hemorrhage. J Urol 2010;183:1947-53. [Crossref] [PubMed]
- Ginat DT, Saad WE, Turba UC. Transcatheter renal artery embolization for management of renal and adrenal tumors. Tech Vasc Interv Radiol 2010;13:75-88. [Crossref] [PubMed]
- Hague J, Tippett R. Endovascular techniques in palliative care. Clin Oncol (R Coll Radiol) 2010;22:771-80. [Crossref] [PubMed]
- Kähler KC, Egberts F, Gutzmer R. Palliative treatment of skin metastases in dermato-oncology. J Dtsch Dermatol Ges 2013;11:1041-5. [Crossref] [PubMed]
- Kvale PA, Simoff M, Prakash UB, et al. Lung cancer. Palliative care. Chest 2003;123:284S-311S. [Crossref] [PubMed]
- Wang GR, Ensor JE, Gupta S, et al. Bronchial artery embolization for the management of hemoptysis in oncology patients: Utility and prognostic factors. J Vasc Interv Radiol 2009;20:722-9. [Crossref] [PubMed]
- Macbeth FR, Wheldon TE, Girling DJ, et al. Radiation myelopathy: Estimates of risk in 1048 patients in three randomized trials of palliative radiotherapy for non-small cell lung cancer. The Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:176-81. [Crossref] [PubMed]
- Spanos WJ Jr, Wasserman T, Meoz R, et al. Palliation of advanced pelvic malignant disease with large fraction pelvic radiation and misonidazole: Final report of RTOG phase I/II study. Int J Radiat Oncol Biol Phys 1987;13:1479-82. [Crossref] [PubMed]
- Chaw CL, Niblock PG, Chaw CS, et al. The role of palliative radiotherapy for haemostasis in unresectable gastric cancer: A single-institution experience. Ecancermedicalscience 2014;8:384. [PubMed]
- Cameron MG, Kersten C, Vistad I, et al. Palliative pelvic radiotherapy of symptomatic incurable rectal cancer - a systematic review. Acta Oncol 2014;53:164-73. [Crossref] [PubMed]
- Johnstone C, Lutz ST. The role of hypofractionated radiation in the management of non-osseous metastatic or uncontrolled local cancer. Ann Palliat Med 2014;3:291-303. [PubMed]
- Abt D, Bywater M, Engeler DS, et al. Therapeutic options for intractable hematuria in advanced bladder cancer. Int J Urol 2013;20:651-60. [Crossref] [PubMed]


