Shedding light on the fundamental mechanism underlying hypnotic analgesia
Introduction
Our conscious experiences encompass a huge variety of phenomenal qualities, ranging from pleasant sensations and emotions at the one end of the spectrum to terrible suffering and agonizing pain on the opposite side. In order to relieve suffering and improve the quality of life of those living with chronic afflictions, clinical hypnosis has gained increasing importance in pain therapy and palliative care. Randomized controlled studies with clinical populations demonstrated that hypnotic suggestions have substantial impacts on pain conditions and analgesia (1,2), indicating that hypnosis is an effective procedure for alleviating pain perception in many different clinical circumstances (3,4).
As a result of the growing interest in clinical hypnosis, the exploration of the underlying mechanism has been considerably intensified. The corresponding research initiatives fit seamlessly into the overarching endeavor to uncover the mechanism behind conscious processes and develop a theory of consciousness. Such a theory is eagerly awaited since it will enable us to understand causal relationships and make valid predictions about the phenomenal properties of conscious systems.
This paper pursues the goal of shedding light on the mechanism underlying conscious perception in general and hypnotic analgesia in particular. The first part of the article deals with the neurophysiological body of evidence and the understanding of the mechanism at the level of neuroscience. As will become apparent, the experience of pain requires the synchronization of spatially divided brain regions and the emergence of coherent large-scale activity patterns, the formation of which is inhibited during the hypnotic state. Building on this, the second part of the article strives for an even deeper understanding of hypnosis on the basis of a more profound theoretical approach to consciousness. As explained in detail below, the empirical findings are compatible with the notion of the brain as a filter that extracts phenomenal nuances selectively from a ubiquitous background field of consciousness. From this perspective, hypnotic analgesia is due to an altered operating mode of the brain that involves an impairment of the filtering mechanism. The article concludes with a brief discussion of the presented approach.
Neurophysiological mechanism underlying hypnotic analgesia
To start with, we take a closer look at the latest evidence from neuroscience. The prevailing strategy behind the constantly evolving activities in this research domain amounts to distilling the neural correlates of consciousness (NCC) and exposing the neurophysiological mechanism underlying consciousness (5,6). In a nutshell, the empirical findings increasingly indicate that conscious states are accompanied by large-scale coherence in the brain, concretely by highly synchronized activity in the beta and gamma frequency bands. Over the last years, various experimental paradigms have confirmed this close connection between large-scale synchronization and consciousness (7-12). As a consequence, the neurophysiological point of view ascribes the unified nature of conscious experience to the binding of distributed neurons into functionally coherent assemblies (6). Moreover, the experimental insights lead to the conclusion that the integration and disintegration of these assemblies is coupled to the theta cycle, with transient gamma-band synchronization of the involved neurons giving rise to a discrete moment of perceptual experience in every period of the cycle (13). This suggests that the stream of consciousness is structured by theta oscillations and that the content of consciousness is modified every few hundred milliseconds (6).
Further studies in animals demonstrate that the macroscopic patterns of synchronized activity in the gamma and beta frequency range represent attractors (i.e., dynamically stable states) in an attractor landscape (14,15). Due to the high correlation of these activity patterns with the “actions and inferred perceptions of the animals” it stands to reason that attractors are the NCC (16). A very important discovery in this context is that “vast collections of neurons shift abruptly and simultaneously” between different attractors (14), leading to the conclusion that the brain is obviously a complex system that operates near a critical point of a phase transition. While the disordered (unconscious) phase exhibits spontaneous activity and irregular dynamics, the ordered (conscious) phase is characterized by long-range correlations and attractors, with an appropriate stimulus being able to induce a transition from the disordered to the ordered phase (16). In addition to these quite revealing results, an even deeper analysis of the data unveils that the background activity of the brain displays so-called null spikes, which are steep decreases in the analytic power occurring at theta rates (17). A null spike triggers a phase transition by dissolving a coherent assembly and causing a short period of disorder, which creates the preconditions for a stimulus to induce a reorganization of the background activity and to initiate the development of the subsequent attractor (17,18).
Taken all together, our normal streams of consciousness are based on the recurring formation and dissolution of ordered brain states that are characterized by a high degree of coherence between spatially distributed cortical areas. This principle applies also to pain, the experience of which requires that “many brain structures must be activated in a temporally correlated manner” (19).
Regarding the neural mechanism underlying hypnotic analgesia, the explanatory approaches assume the involvement of an altered state of consciousness, “which would imply that hypnosis leads to an objectively measurable change in the functioning of the brain that cannot be explained by ordinary psychological mechanisms, such as selective attention” (20). This assumption is supported by the neurophysiological findings, according to which beta- and gamma-generated assemblies in the whole cortex exhibit decreased size and decreased stability during the hypnotic state, thus revealing considerable differences between baseline and hypnotic conditions (20,21). The effects are particularly pronounced in high hypnotizable subjects who show “significant reductions in phase-ordered gamma patterns… during hypnosis and post-hypnosis conditions” (22,23) and decreased functional connectivity in the beta frequency band (24). These insights indicate that hypnotic analgesia is characterized by a loss of coherence between brain areas, reflecting “an alteration or even a breakdown of communication between the subunits of the brain” (19).
In summary, the neurophysiological findings suggest that the hypnotic state is associated with an altered operating mode that is clearly different from the normal operating mode of the brain. While in the normal operating mode a dolorogenic stimulus induces a large-scale activity pattern (i.e., an attractor) that leads to the experience of pain, the altered operating mode inhibits the synchronization of spatially divided brain regions, resulting in a breakdown of coherent large-scale cortical oscillations. As a consequence, attractors cannot develop and the conscious experience of pain cannot arise. These interdependencies are depicted in Figure 1.
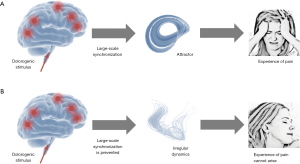
Fundamental mechanism underlying hypnotic analgesia
In the previous section, we discussed the neurophysiological mechanisms behind normal conscious states and hypnotic states. Unquestionably, this discussion provided valuable insights into the way hypnosis influences the brain processes underlying conscious perception. However, viewed from the perspective of theoretical physics, it seems absolutely implausible that a basic understanding of consciousness, including altered states of consciousness, can be achieved at the description level of neural networks. Rather, the elaboration of a truly fundamental theory of consciousness can only be successful at the lowest description level that offers the possibility to discover the basic principles behind conscious systems and relate these principles to the fundamental interactions and force fields that form the foundations of physics. The establishment of such an interrelationship is an essential prerequisite for a seamless integration of consciousness into our scientific worldview (25-27).
Interestingly, stochastic electrodynamics (SED), a branch of physics that pursues the goal of unveiling the origin of quantum phenomena, turns out to be a suitable framework and promising starting point for a fundamental theory of consciousness. The significant progress that can be attributed to SED consists in the provision of novel insight into the physical mechanisms behind quantum systems, thus paving the way for a deeper understanding of quantum physics (28-35). At its heart, SED is based on the notion that the universe is imbued with an all-pervasive electromagnetic background field, called zero-point field (ZPF), which can be viewed as a sea of light featuring a unique frequency spectrum. The undisturbed ZPF is a stochastic field with completely uncorrelated field modes (28,29,33).
According to SED, the ZPF interacts permanently with the electrically charged components of every physical system, thus inducing stochastic oscillations in the system components. As long as disturbing forces such as thermal noise are negligible compared to the coupling strength between the charged components and the relevant field modes, the energy exchange between the components and the ZPF reaches a state of dynamic equilibrium in which the average radiated power is exactly equal to the average power absorbed by the system. These balance situations fulfill quantization conditions in accordance with the stationary states predicted by quantum theory (29,31,32). As a consequence, any dynamical system in energetic balance with the ZPF exhibits quantum behavior. Upon reaching equilibrium, a quantum system falls into an attractor (29), or put differently, enters a dynamically stable state orchestrated by the ZPF.
Since matter and ZPF are closely intertwined, the interplay with the charged components of a quantum system influences the dynamics of the ZPF in such a way that the relevant ZPF modes, which play the role of a system-specific set of resonance frequencies bringing about the maintenance of the equilibrium, become highly correlated and enter a state of phase-locked coupling (32). This means that the originally disordered ZPF undergoes a transition and changes into a partially organized field as soon as a quantum system falls into an attractor (33). As a result, all the system components are in tune with the modified ZPF (31), which is the root cause for long-range coherence and collective cooperation.
In summary, the key concept behind the theoretical framework of SED is the idea of a ubiquitous background field that acts as a creative agent and gives birth to the enormous variety of material manifestations and island of stability in the universe. The insights provided by SED imply that matter and ZPF are part of a mutual shaping and orchestration process, in the course of which on the one hand stable structures and on the other hand ordered ZPF states arise. This indicates that the basic components of matter are devoid of intrinsic physical properties. Rather, their properties originate from a dynamic interaction process with the background field. Consequently, quantum phenomena can be understood as emergent phenomena that can be attributed to the resonant interaction between the components of matter and the ZPF.
These core principles underlying quantum systems may be interpreted in two ways (27). For one thing, the phase-locked ZPF modes accompanying the formation of an attractor are equivalent to an information state in the background field, subsequently referred to as a ZPF information state. Since each quantum system is characterized by a unique set of resonance frequencies and, hence, a distinctive pattern of phase-locked ZPF modes, the content of such an information state is determined by the dynamical properties of the corresponding attractor. For another thing, the mechanism described above may be construed as a particular kind of extraction or filtering, which is due to the functioning of a quantum system as a stochastic oscillator selectively picking off its set of resonance frequencies from the frequency spectrum of the ZPF. Accordingly, the formation of an attractor goes along with the selective extraction and phase locking of the system-specific ZPF modes.
Given these explications, the zero-point field commends itself as a perfect candidate for the substrate of consciousness. Consequently, I posit that all conceivable nuances of phenomenal awareness are woven into the fabric of the ZPF. Moreover, it seems reasonable to postulate identity between the mechanism underlying quantum systems and the fundamental mechanism underlying conscious systems, implying that the core principle behind conscious systems relies on the creation of order in the omnipresent substrate of consciousness and that every generated ZPF information state has an internal representation manifesting itself as a conscious state. This approach rests upon the notion of the ZPF as a fundamental field that plays a dual role as the carrier of energy and consciousness, suggesting that one and the same mechanism is at work with regard to the physical properties and phenomenal qualities of quantum systems. To be more precise, the principle of dynamical coupling of sets of ZPF modes promises to be eminently suitable for the extraction of an enormous variety of phenomenal shades from the phenomenal color palette defined by the ZPF (27).
Do conscious brain processes actually rest upon the proposed mechanism? Coming back to the neurophysiological body of evidence, the findings compiled in the previous section clearly imply that the characteristic features of macroscopic brain dynamics, such as phase transitions, pattern formation, and dissipative structures, cannot be sufficiently explained on the basis of classical physics and, hence, require an explanatory approach resorting to quantum physics (36,37). This means that SED provides the appropriate framework to describe the dynamical properties of the NCC, most notably their rapid formation and dissolution as well as their enormous coherence length.
From the perspective of SED, the cortical background activity can be traced back to the interaction between the cortex and the ZPF. Induced by an appropriate sensory stimulus, a reorganization of the background activity accompanied by a phase transition and attractor formation takes place, which in turn crucially involves the ZPF as a stabilizing agent and communication medium that synchronizes all sections of the attractor-related coherence domain. The result of this mutual shaping process is a reorganization of the ZPF, once a transiently stable equilibrium between the synchronously oscillating cell assembly and the ZPF is established. As explained above, such a reorganization of the ZPF can be interpreted as a ZPF information state that is on the one hand characterized by an attractor-specific pattern of phase-locked ZPF modes and on the other hand expected to represent a conscious state. As the process continues, a null spike interrupts the coupling between the oscillating cell assembly and the ZPF, thus triggering the dissolution of the existing attractor and preparing the ground for the development of the subsequent attractor. In this manner, the brain produces an individual stream of consciousness by periodically generating ZPF information states (26,27).
We can conclude that SED manages to derive a consistent overall picture from the neurophysiological discoveries pertaining to conscious processes. The uniqueness of attractors lies in their access to the ZPF, the presumed substrate of consciousness. Hence, the body of evidence supports the view that in the normal operating mode our brains act as filters that extract phenomenal nuances selectively from the ubiquitous background field of consciousness (26,27).
These findings open the door to a deeper understanding of hypnosis and unveil the fundamental mechanism underlying hypnotic analgesia. The crucial insight is that in the normal operating mode a dolorogenic stimulus induces an attractor that is accompanied by an attractor-specific ZPF information state, which leads to the experience of pain. In contrast, the altered operating mode, which establishes under hypnotic conditions, inhibits the synchronization of spatially divided brain regions, thus preventing the formation of attractors and concomitant ZPF information states. As a consequence, the conscious experience of pain cannot arise. These causal relationships are depicted in Figure 2.
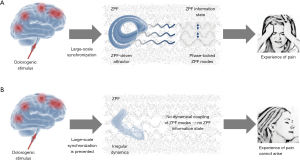
Discussion and conclusions
In the previous sections, we discussed a novel approach to the scientific understanding of consciousness and obtained a clear demarcation criterion between unconscious and conscious brain processes in such a way that only those processes that cause local modifications of the ZPF are accompanied by conscious experiences. This leads to a modern interpretation of hypnosis, according to which during hypnotic conditions the normal interaction mechanism between the brain and the ZPF is impaired and stimuli are excluded from conscious awareness that are normally consciously perceived.
The approach presented in this article displays all the essential characteristics that are expected from an advanced explanatory framework for consciousness. It is comprehensive, conceptually coherent, and supported by empirical evidence. Most notably, it obeys the principle of causal closure and it is able to reconcile phenomenal awareness with the laws of nature. Expressed even more precisely, the framework reveals the dynamical key features of conscious systems and sheds light on the conditions under which a given system can have conscious experiences. This suggests that the body of thought put forward in this article lays a solid foundation for a theory of consciousness, the development of which will promote the systematic study of conscious states and open up new horizons for medical fields of application.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Montgomery GH, David D, Winkel G, et al. The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesthesia & Analgesia 2002;94:1639-45. [PubMed]
- Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull 2003;129:495-521. [Crossref] [PubMed]
- Vanhaudenhuyse A, Boly M, Laureys S, et al. Neurophysiological correlates of hypnotic analgesia. Contemp Hypn 2009;26:15-23. [Crossref]
- Brugnoli MP. Clinical hypnosis for palliative care in severe chronic diseases: a review and the procedures for relieving physical, psychological and spiritual symptoms. Ann Palliat Med 2016;5:280-97. [Crossref] [PubMed]
- Aru J, Bachmann T, Singer W, et al. Distilling the neural correlates of consciousness. Neurosci Biobehav Rev 2012;36:737-46. [Crossref] [PubMed]
- Singer W. The ongoing search for the neuronal correlate of consciousness. In: Metzinger T, Windt JM. editors. Open MIND. MIND Group, Frankfurt am Main, 2015;36.
- Crick F, Koch C. Towards a neurobiological theory of consciousness. Sem Neurosci 1990;2:263-75.
- Desmedt JE, Tomberg C. Transient phase-locking of 40 Hz electrical oscillations in prefrontal parietal cortex reflects the process of conscious somatic perception. Neurosci Lett 1994;168:126-9. [Crossref] [PubMed]
- Rodriguez E, George N, Lachaux JP, et al. Perception’s shadow: long distance synchronization of human brain activity. Nature 1999;397:430-3. [Crossref] [PubMed]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 2001;5:16-25. [Crossref] [PubMed]
- Melloni L, Molina C, Pena M, et al. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci 2007;27:2858-65. [Crossref] [PubMed]
- Gaillard R, Dehaene S, Adam C, et al. Converging intracranial markers of conscious access. PLoS Biol 2009;7:e61. [Crossref] [PubMed]
- Doesburg SM, Green JJ, McDonald JJ, et al. Rhythms of consciousness: binocular rivalry reveals large-scale oscillatory network dynamics mediating visual perception. PLoS One 2009;4:e6142. [Crossref] [PubMed]
- Freeman WJ. The physiology of perception. Scientific American 1991;264:78-85. [Crossref] [PubMed]
- Freeman WJ. Origin, structure, and role of background EEG activity. Part 3. Neural frame classification. Clin Neurophysiol 2005;116:1118-29. [Crossref] [PubMed]
- Freeman WJ. Indirect biological measures of consciousness from field studies of brains as dynamical systems. Neural Networks 2007;20:1021-31. [Crossref] [PubMed]
- Freeman WJ. Deep analysis of perception through dynamic structures that emerge in cortical activity from self-regulated noise. Cogn Neurodyn 2009;3:105-16. [Crossref] [PubMed]
- Freeman WJ. Origin, structure, and role of background EEG activity. Part 1. Analytic amplitude. Clin Neurophysiol 2004;115:2077-88. [Crossref] [PubMed]
- Miltner WH, Weiss T. Cortical mechanisms of hypnotic pain control. In: Jamieson GA. editor. Hypnosis and Conscious States. The Cognitive Neuroscience Perspective. Oxford: Oxford University Press, 2007:51-66.
- Fingelkurts AA, Fingelkurts AA, Kallio S, et al. Cortex functional connectivity as a neurophysiological correlate of hypnosis: an EEG case study. Neuropsychologia 2007;45:1452-62. [Crossref] [PubMed]
- Kallio S, Revonsuo A. Hypnotic phenomena and altered states of consciousness: a multilevel framework of description and explanation. Contemp Hypn 2003;20:111-64. [Crossref]
- De Pascalis V, Cacace I, Massicolle F. Perception and modulation of pain in waking and hypnosis: functional significance of phase-ordered gamma oscillations. Pain 2004;112:27-36. [Crossref] [PubMed]
- De Pascalis V. Phase-ordered gamma oscillations and modulation of hypnotic experience. In: Jamieson GA. editor. Hypnosis and Conscious States: The Cognitive Neuroscience Perspective. Oxford: Oxford University Press, 2007:67-89.
- Jamieson GA, Burgess AP. Hypnotic induction is followed by state-like changes in the organization of EEG functional connectivity in the theta and beta frequency bands in high-hypnotically susceptible individuals. Front Hum Neurosci 2014;8:528. [Crossref] [PubMed]
- Keppler J. A conceptual framework for consciousness based on a deep understanding of matter. Philos Study 2012;2:689-703.
- Keppler J. A new perspective on the functioning of the brain and the mechanisms behind conscious processes. Front Psychol 2013;4:242. [Crossref] [PubMed]
- Keppler J. On the universal mechanism underlying conscious systems and the foundations for a theory of consciousness. Open J Phil 2016;6:346-67. [Crossref]
- De la Peña L, Cetto AM. Quantum phenomena and the zeropoint radiation field. Found Phys 1994;24:917-48. [Crossref]
- De la Peña L, Cetto AM. Quantum phenomena and the zeropoint radiation field II. Found Phys 1995;25:573-604. [Crossref]
- De la Peña L, Cetto AM. The Quantum Dice: An Introduction to Stochastic Electrodynamics. Dordrecht: Kluwer Academic Publishers, 1996.
- De la Peña L, Cetto AM. Quantum theory and linear stochastic electrodynamics. Found Phys 2001;31:1703-31. [Crossref]
- De la Peña L, Cetto AM. The foundations of linear stochastic electrodynamics. Found Phys 2006;36:350-68. [Crossref]
- De la Peña L, Valdés-Hernández A, Cetto AM. Quantum mechanics as an emergent property of ergodic systems embedded in the zero-point radiation field. Found Phys 2009;39:1240. [Crossref]
- Cetto AM, De la Peña L, Valdés-Hernández A. Quantization as an emergent phenomenon due to matter-zeropoint field interaction. J Phys Conf Ser 2012;361:012013. [Crossref]
- De la Peña L, Cetto AM, Valdés-Hernández A. The Emerging Quantum: The Physics Behind Quantum Mechanics. Cham: Springer International Publishing, 2015.
- Freeman WJ, Vitiello G. Nonlinear brain dynamics as macroscopic manifestation of underlying many-body field dynamics. Phys Life Rev 2006;3:93-118. [Crossref]
- Freeman WJ, Vitiello G. The dissipative quantum model of brain and laboratory observations. Electr J Theor Phys 2007;4:1-18.


