Radiotherapy for brain metastases near the end of life in an integrated health care system
Introduction
Lung carcinoma remains the leading cause of cancer death worldwide (1,2). Brain metastasis (BrM) are expected in 20–30% of patients (3,4), with prevalence likely to grow over time given the advent of more effective local and systemic therapies as well as increasingly sensitive imaging modalities that improve early/subclinical detection (5).
With over half of metastatic lung cancer patients at some point receiving palliative radiotherapy (RT) (6), the current and projected burden of BrM upon patients, caregivers, and health services is non-trivial. Brain RT has long been considered a standard of care (7,8); its receipt has been adopted as a quality indicator by the Veterans Health Administration (9), and it has been a focus for timely intervention by the Rapid Response Radiotherapy Program in Canada, where 54% of patients started brain RT on the day of consultation (10).
However, in a group bearing relatively poor prognosis (8,11,12), over-treatment and aggressive care at the end of life (EOL) pose questions of concern since the purported benefits of RT may not be realized when life expectancy is short, as demonstrated recently by the Quality of Life after Treatment for Brain Metastases (QUARTZ) trial (5,13,14). Poor prognostication on the part of providers, inaccurate perceptions of disease curability by patients, and overly optimistic expectations of RT by referring physicians (15-18) may, in part, explain increasing use of RT for metastatic lung cancer within days of death (14,15,19-22). While these accounts raise the spectre of poor quality, akin to measures of inappropriate EOL cancer care (23,24), whether similar indications and time frames apply to RT is less clear (18-19,22).
The intuition that RT received within days of death constitutes suboptimal care warrants not only evaluation of treatment outcomes, but better understanding of health services that BrM patients receive—processes not well-described in the literature, especially with regard to events leading to radiation treatment (20). In an effort to parse these patterns-of-care, we assessed BrM incidence among non-small cell lung cancer (NSCLC) patients diagnosed within an integrated, multi-facility health care system of a diverse metropolitan area and observed referral, consultation, and treatment rates. To gain further insight on RT near the EOL, we identified those at highest short-term risk of death as predicted by diagnosis-specific Graded Prognostic Assessment (GPA) (11) score of 0–1 (median survival 3 months) to evaluate whether this would impact RT receipt within 14 or 30 days of death.
Methods
All incident NSCLC cases diagnosed in 2007–2011 were identified by cancer registry of the Kaiser Permanente Southern California integrated healthcare system, which retains enrollees for an average of 14 years (25). BrM were determined by reports on all imaging performed from 1 month prior to diagnosis up to 03/31/2013 and confirmed by clinical documentation in the electronic health record (EHR).
Data collection
Demographic information and vital statistics up to 03/31/2015 were obtained through clinical and administrative data collected by Kaiser Permanente; minimum follow-up was 4.25 years from NSCLC diagnosis and 2 years from BrM diagnosis. Disease histology and stage were established by cancer registry. Other tumor, clinical, treatment, and health services information was abstracted manually from the EHR (JJR).
Health services
Rates of referral to radiation oncology (RO), consultation fulfillment, recommendation for brain RT, and receipt of RT were assessed. Documented reasons were examined for lack of referral, lack of consultation, recommendation against brain RT, and lack of recommended RT receipt. While one internal RO department serves the regional healthcare system, private radiation facilities are contracted for services to patients at the metropolitan margins, where distance may be a barrier to provider access and treatment; external RO consultation and treatment were also examined. Treatment factors included type of RT [whole brain (WBRT) or stereotactic radiosurgery (SRS)], planned number of radiation fractions, and whether RT was completed as planned.
Outcomes
Treatment near the EOL was measured from date of last RT fraction received to date of death, with examination of radiation receipt within 14 and 30 days of death.
Covariates
Patient characteristics included age at NSCLC and at BrM diagnosis, sex, race/ethnicity, marital status, and median income for residence census block. Charlson comorbidity score was derived by inpatient diagnosis coding from administratively collected data (26). Tumor/clinical characteristics included group stage at NSCLC diagnosis, number of BrM lesions, primary disease control at time of BrM diagnosis, number of extracranial metastatic sites, and presence of liver metastases. Performance status (PS) was categorized as good (ECOG PS 0–1, Karnofsky score 90–100%, qualitative statement of “excellent” or “good”), fair (ECOG PS 2, Karnofsky score 70−80%, qualitative statement of “fair”), poor (ECOG PS 3–4, Karnofsky score <70%, qualitative statement of “poor”), or unknown. Other factors included advanced directives and/or Do Not Resuscitate/Do Not Intubate (DNR/DNI) code status established prior to RO consultation, RO consultation while hospitalized, and RO consultation/RT received at external RO facilities.
GPA
NSCLC-specific GPA score based on age, PS, presence/absence of extracranial metastasis, and number of BrM lesions was derived based on collected data (for example: age >60 years, poor PS, extracranial metastasis, and >3 BrM lesions would yield a score of 0) (11). Initially developed from a Radiation Therapy Oncology Group (RTOG)database analysis and validated in an independent cohort of BrM patients, the GPA predicts poorest median survival of 3 months when the score is 0−1. GPA score 0−1 was used as a covariate in analyses.
Statistical analyses
Bivariate analyses of outcomes were performed by all covariates. Multivariate logistic regression models for death within 14 and 30 days of RT were developed for BrM patients who received at least one fraction of radiation. Covariates significantly correlated with dependent variables in bivariate analyses or deemed requisite for adjustment from conceptual standpoints were included in the models. All analyses were performed using Stata statistical software, version 13.1 (College Station, TX, USA).
This study was approved by the Kaiser Permanente Southern California Institutional Review Board (No. 6434).
Results
Of 5,133 NSCLC patients, median age at diagnosis was 71 years (SD 10.8), 52% were male, 63% white, and 56% had stage IV disease at diagnosis. BrM were found on presentation in 10%, while 7% developed BrM subsequently. At minimum follow-up of 4.25 years from diagnosis, 83% of patients had died.
Referral patterns
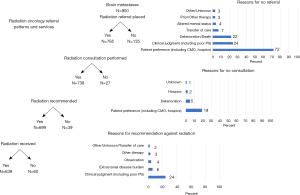
Among 900 patients with BrM, 135 (15%) were not referred to RO for reasons such as patient preference (n=68), deterioration/death (n=28), or clinical judgments (n=26) (Figure 1). Post-operative referrals were made for 71 (8%); RO consultation was not fulfilled in 26 (3%) due to patient preference (n=19) or deterioration/death (n=7). Among 738 patients who received RO consultation, 39 (5%) were not recommended RT, largely based on clinical factors/judgments (n=30); 7 patients received RT despite this. In total, 639 patients received RT (91 of whom received >1 episode of treatment): 597 patients WBRT (17 on >1 occasion), 126 SRS (30 on >1 occasion), and 54 both WBRT and SRS.
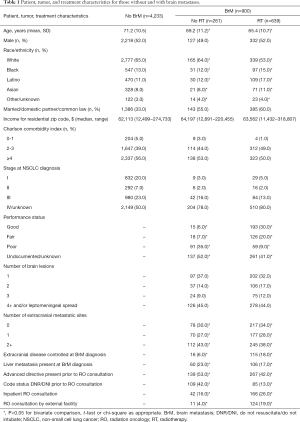
Patient characteristics
In this racially diverse sample, non-white patients were significantly more likely to receive RT than whites (Table 1). Among those receiving RT, PS was better, single brain lesions more prevalent, metastatic sites fewer, and extracranial disease more often controlled, while the proportion of patients with liver metastases and advanced directives or DNR/DNI code status established prior to RO consultation lower among those receiving RT (Table 1).
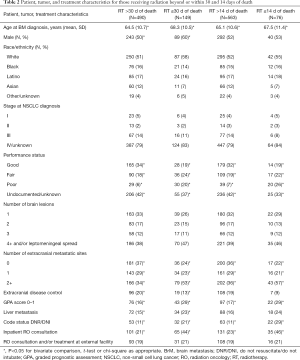
Full table
EOL RT
Of 639 patients receiving at least one fraction of RT, 11.9% died within 14 days and 23.3% (cumulatively) within 30 days of last treatment. SRS was received by 2 patients dying within 14 days and 3 within 15–30 days of RT. Poorer PS, increasing metastatic sites, establishment of DNR/DNI code status prior to RO consultation, and inpatient RO consultation were significantly more likely among those dying within 14 or 30 days of RT than among those surviving beyond those timeframes (Table 2). In addition, those dying within 30 days of RT receipt were more likely to have uncontrolled extracranial disease and liver metastasis.
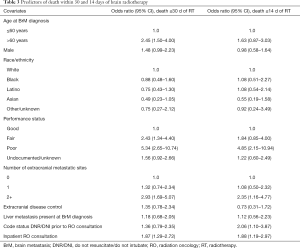
Full table
On multivariate analysis, poor PS, ≥2 extracranial metastatic sites, DNR/DNI code status established prior to RO consultation, and RO consultation while hospitalized incurred higher odds of death within 14 days of RT (Table 3). Similarly, poor PS, ≥2 extracranial metastatic sites, and inpatient RO consultation predicted for higher odds of death within 30 days of RT receipt, as did age >60 years and fair PS (Table 3).
Full table
Treatment completion and planned fractionation
Among 50 patients who did not complete RT as planned, mean time from RT to death was 19 days (SD 35); 32% dying within 14 days and 39% within 30 days of RT did not complete treatment as planned. Reasons for incomplete treatment were death in 42%, deterioration in 34%, and patient preference in 14%; 1 patient ceased treatment due to disease progression.
Of those whose last treatment was WBRT, 363 (65%) were planned for 10 fractions and 121 (22%) for 5. Mean time from RT to death was shorter when 5 vs. 10 fractions were planned (78 vs. 179 days, P<0.01).
While the maximum number of planned WBRT fractions was 15 among those with internal RO providers, 10 of 129 patients with external RO providers were planned for 16–25 fractions. Though at external RO facilities, the number of planned fractions was higher and patients less likely to complete RT as planned (P<0.01), the number of deaths during treatment and near the EOL was not significantly different from those treated within the healthcare system on multivariate analysis.
Survival
Mean time to death from date of BrM diagnosis for patients: referred and not referred for RT was 208 days (95% CI: 190–227) vs. 43 days (95% CI: 31–55), P<0.001; receiving and not receiving RO consultation was 214 days (95% CI: 195–233) vs. 48 days (95% CI: 35–61), P<0.001; receiving and not receiving brain RT was 231 days (95% CI: 209–252) vs. 69 days (95% CI: 56–83), P<0.001. Median time from BrM diagnosis to death for those advised against RT was 48 days (range, 13–764 days).
Among 135 patients not referred for brain RT, the decision to forego referral was made during hospital admission for 72% and mean time from BrM to death was 43 days (SD 67). For 39 patients not recommended RT on consultation, mean time from BrM diagnosis to death was 96 days (SD 139).
GPA
Based on available information, it was possible to determine that at least 202 (22%) patients bore a GPA score of 0–1, corresponding to median survival of 3 months by RTOG patients from whom the classification was derived (11). Patients of GPA score 0–1 in our study had a median survival of 49 days (range, 0–1,188 days). Of these patients, 119 (59%) received RT, 22 (11%) within 14 days and 43 (21%) within 30 days of death. On adjustment, GPA score 0–1 was significantly associated with death ≤30 days of RT; male sex, liver metastasis, DNR/DNI code status established prior to consultation, and inpatient RO consultation were also independent predictors of death within 30 days of RT (Table 4).

Full table
Discussion
In this diverse cohort of NSCLC patients, BrM were present at diagnosis or developed in 17%. This resulted in referrals for over 700 patients during the follow-up period and many recommendations for brain RT.
Alarmingly, close to 12% of the 639 patients who received at least one fraction of RT died within 14 days of treatment, while 23% died within 30 days of treatment.
As trends in EOL care grow increasingly aggressive (19,20,23,24,27), striking the balance between palliation and overtreatment in a patient’s final days is imperative. Quality measures have sought to discourage use of chemotherapy within 14 days of death, while researchers in RO have pointed to RT within 14 to 30 days of death or greater than 10% of remaining lifespan spent in treatment as potential indications of poor quality care (19-22,27).
When palliation is the goal of treatment, however, and patients are by nature of disease near the EOL, time standards are difficult to define. On the one hand, there have been reasonable concerns for underuse of an efficacious modality to palliate symptoms and improve quality of life (28,29), such as the National Hospice Study revealing <3% of patients received RT despite over half of their surveyed providers indicating BrM merited RO referral (30). On the other, poor prognosis and outcomes of BrM patients with or without treatment has led to Phase III comparison of brain RT versus best supportive care (QUARTZ trial) (5).
Given frequencies in the literature ranging from 1% of patients dying within 14 days (14) and 17% within 6 weeks of RT initiation (31), our findings may signal overuse that proffers a significant opportunity for improvement. As survival of patients treated near the EOL is comparable to that of the 15% not referred for RT (largely for clinical and patient preference reasons that seem appropriate given mean time from BrM to death of 43 days), underutilization of RT does not appear as problematic in our system.
Of interest, 72% of decisions to forego referral were made during patient hospitalization. Though decisions in hospital not to refer patients seem apropos, decisions to offer RT after evaluation in that same setting should give pause based on our experience, as such was associated with a 2-fold increase in odds for death within 14 or 30 days of treatment. This may reflect greater inaccuracy in assessing life expectancy when patients bear acute conditions requiring admission and may speak to value in the outpatient “litmus test” of ability to undergo not only treatment itself, but its often taxing logistics.
Other predictors of EOL RT in our analysis, such as poor PS and increasing metastatic sites, are in keeping with well-established prognosticators for limited survival. Though we found GPA not once explicitly documented in EHR review, GPA score 0–1 derived from collected data showed at least one-fifth of patients fell in this category, with median survival of 49 days from BrM discovery. Over one-quarter of those dying within 14 or 30 days of treatment bore GPA score 0–1. Further supporting the prognostic accuracy of GPA classification in the real-world clinical setting, GPA score 0–1 predicted for receipt of RT within 30 days of death.
With availability of validated prognostication tools such as the GPA (11), decisions for treatment and fractionation theoretically should be made with greater objectivity and lend to improved outcomes. However, as the growing body of literature suggests, accurate estimations of life expectancy and treatment efficacy still remain elusive to referring providers, patients, and radiation oncologists themselves (15-18). The optimism is tremendous: 87% of surveyed referring providers believed WBRT would improve PS and 41% that it would increase survival (18); 78% of surveyed metastatic lung cancer patients believed palliative RT would prolong life (17); and 67% of surveyed radiation oncologists overestimated life expectancy despite recognizing that PS, BrM, and primary site are significant prognosticators (16). In another study of patients who died within 30 days of evaluation for palliative RT, life expectancy was accurately predicted by radiation oncologists in only 16%, while that of 21% was overestimated by >6 months (15). Only 26% of patients in this study indicated that symptoms were alleviated with RT and 52% reported progressive complaints after RT—striking since median treatment time “resemble(d) median survival time” (15). These findings are supported by a systematic review of WBRT clinical trials showing mixed results on whether quality of life improved with treatment (32), as well as by the QUARTZ trial showing best supportive care to be non-inferior to WBRT with regard to quality-adjusted life-years (5).
Despite the frequency of EOL RT in our study, signs of clinical acumen were not altogether absent. For the 5% advised against RT, median time from BrM to death was 48 days, which sits comfortably below the 3-month life-expectancy cutoff suggested by some for consideration of palliative RT (33,34). Mean time from RT to death was also, perhaps, acceptable (<10% remaining days in treatment) and shorter when 5 vs. 10 fractions were planned (78 vs. 179 days, P<0.01), intimating that providers had a sense of prognosis.
Undoubtedly, it is challenging for providers to make accurate assessments and deliver difficult news, especially when best supportive care is perceived as a loss of hope (18), but as Chen et al. astutely remark, measured deliberation of treatment is paramount as “we may also put many patients with limited prognosis through more treatment than necessary, which can lead to undue burden on families, unnecessary adverse effects, and excessive costs to an already-stretched health care system, with unclear benefits” (35). Greater heed with regard to radiation recommendations for the NSCLC population is crucial, and benchmarks for quality of palliative radiation care are sorely needed.
In reporting EOL RT outcomes, this study aims to provide more evidence in support of creating such benchmarks, but it bears limitations. Being retrospective, data are restricted to documentation in the medical record; inconsistency across providers was observed for prognostic factors such as PS, which appeared well-documented only when exceptionally good or exceptionally poor. Information regarding quality of life and cause of death was not always available, so conclusions could not be made regarding other important outcomes associated with RT. Pathological features affecting prognosis, such as EGFR mutation status, were not accessible. Drawing patients and care patterns from an integrated health care system, this study may not generalize well to the community at large given the uncommon setting in which no financial incentive exists to treat at the provider level. That said, the population served is diverse in age and race/ethnicity; coupled with minimal loss to follow-up and lengthy follow-up time, these are important strengths, in addition to insight gained on reasons for healthcare decisions made by providers (referral, consultation, RT recommendation) and patients (acceptance of referral, consultation, and RT). Future inquiries would benefit from evaluating the role of early palliative care, which could not be assessed, as well as its influence on referral rates and RT recommendations.
In summary, BrM incidence in this sample was 17%, with the majority harboring BrM on initial NSCLC presentation. While 15% were not referred for RT, this appears appropriate given the limited remaining lifespan of these patients who largely declined referral based on preference or were deemed unfit for clinical reasons to undergo treatment. A surprising proportion of patients recommended brain RT was treated near the EOL: 12% died within 14 days and 23% (cumulatively) within 30 days of last treatment. GPA score 0–1 independently predicted for death within 30 days of RT. While palliative therapies by nature may occur near the EOL, careful consideration of radiation recommendation, timing, and fractionation scheme—especially during hospitalization—should aim to minimize the projected proportion of remaining days spent in active treatment and to maximize quality of life. Use of validated prognostication tools for objective measurement of life expectancy should aid in this endeavor as benchmarks for quality of palliative radiation care are explored and developed.
Acknowledgements
Funding: This work was supported by the Kaiser Permanente Southern California Regional Research Committee (Project No. CS112240). A debt of gratitude is owed to John Sim, William Towner, Michael Girvigian, Ricardo Wang, and Michael Tome for their kind assistance and support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Kaiser Permanente Southern California Institutional Review Board (No. 6434).
References
- International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012. , accessed 29 December 2016.http://www.who.int/mediacentre/factsheets/fs297/en/
- Surveillance, Epidemiology, and End Results Program, National Cancer Institute. SEER Stat Fact Sheets. , accessed 29 December 2016.http://seer.cancer.gov/statfacts/html/lungb.html
- Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment. Cancer 2002;95:605-12. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ). Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Hayman JA, Abrahamse PH, Lakhani I, et al. Use of palliative RT among patients with metastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2007;69:1001-7. [Crossref] [PubMed]
- Chi A, Komaki R. Treatment of brain metastasis from lung cancer. Cancers 2010;2:2100-37. [Crossref] [PubMed]
- Stavas M, Arneson K, Friedman J, et al. From whole brain to hospice. J Palliat Med 2014;17:662-6. [Crossref] [PubMed]
- Oishi SM, Antonio AL, Ryoo J, et al. Quality of supportive care for patients with advanced lung cancer in the Veterans Health Administration. J Community Support Oncol 2014;12:361-9. [Crossref] [PubMed]
- Thavarajah N, Wong K, Zhang L, et al. Continued success in providing timely palliative radiation therapy at the Rapid Response Radiotherapy Program. Curr Oncol 2013;20:e206-11. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the Graded Prognostic Assessment. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Yu C, Sloan AE, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro-Oncology 2012;14:910-8. [Crossref] [PubMed]
- Fine PG. Palliative radiation therapy in end-of-life care. Am J Hosp Palliat Care 2002;19:166-70. [Crossref] [PubMed]
- Jung H, Sinnarajah A, Enns B, et al. Managing brain metastases patients with and without RT. Support Care Cancer 2013;21:3379-86. [Crossref] [PubMed]
- Gripp S, Mjartan S, Boelke E, et al. Palliative RT tailored to life expectancy in end-stage cancer patients. Cancer 2010;116:3251-6. [Crossref] [PubMed]
- Tseng YD, Krishnan MS, Sullivan AJ, et al. How radiation oncologists evaluate and incorporate expectancy estimates into the treatment of palliative cancer patients. Int J Radiat Oncol Biol Phys 2013;87:471-8. [Crossref] [PubMed]
- Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol 2013;31:2730-5. [Crossref] [PubMed]
- Barnes EA, Chow E, Tsao MN, et al. Physician expectations of treatment outcomes for patients with brain metastases referred for whole brain RT. Int J Radiat Oncol Biol Phys 2010;76:187-92. [Crossref] [PubMed]
- Kapadia NS, Mamet R, Zornosa C, et al. Radiation therapy at the end of life in patients with incurable nonsmall cell lung cancer. Cancer 2012;118:4339-45. [Crossref] [PubMed]
- Guadagnolo BA, Liao K-P, Elting L, et al. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol 2013;31:80-7. [Crossref] [PubMed]
- Murphy JD, Nelson LM, Chang DR, et al. Patterns of care in palliative RT. J Oncol Practice 2013;9:e220-227. [Crossref]
- Jones JA, Lutz ST, Chow E, et al. Palliative RT at the end of life. CA Cancer J Clin 2014;64:296-310. [PubMed]
- Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 2005;17:505-9. [Crossref] [PubMed]
- Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life. J Clin Oncol 2008;26:3860-6. [Crossref] [PubMed]
- McCarthy D, Mueller K, Wrenn J. Kaiser Permanente: Bridging the quality divide with integrated practice, group accountability, and health information technology. The Commonwealth Fund 2009;17:1-26.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. [Crossref] [PubMed]
- Toole M, Lutz S, Johnston PAS. Radiation oncology quality. J Am Coll Radiol 2012;9:199-202. [Crossref] [PubMed]
- Lavergne MR, Johnston GM, Gao J, et al. Variation in the use of palliative radiotherapy at end of life. Palliat Med 2011;25:101-10. [Crossref] [PubMed]
- Kong W, Jarvis CR, Sutton DS, et al. The use of palliative whole brain radiotherapy in the management of brain metastases. Clin Oncol (R Coll Radiol) 2012;24:e149-158. [Crossref] [PubMed]
- Lutz S, Spence C, Chow E, et al. Survey on use of palliative radiotherapy in hospice care. J Clin Oncol 2004;22:3581-6. [Crossref] [PubMed]
- Windsor AA, Koh ES, Allen S, et al. Poor outcomes after whole brain radiotherapy in patients with brain metastases. Clin Oncol (R Coll Radiol) 2013;25:674-80. [Crossref] [PubMed]
- Wong J, Hird A, Kirou-Mauro A, et al. Quality of life in brain metastases radiation trials. Curr Oncol 2008;15:25-45. [PubMed]
- Craighead PS, Chan A. Defining treatment for brain metastases patients. Support Care Cancer 2012;20:279-85. [Crossref] [PubMed]
- Krishnan MS, Epstein-Peterson Z, Chen YH, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy. Cancer 2014;120:134-41. [Crossref] [PubMed]
- Chen AB, Schrag D. Reply to F Fiorica et al and D Vordermark. J Clin Oncol 2013;31:2759-60. [Crossref] [PubMed]


