The role of cryosurgery in palliative care for cancer
Introduction
Currently, the only curative treatment option for cancer is surgical resection. However, only a small percentage of patients with cancer are eligible for surgery because majority of them cannot receive radical operation due to tumor size and location, and patient’s co-morbidity or poor performance. Particularly over the last decade, attempts to increase the number of treatable cancer at the same time developing less invasive approaches to the destruction of the tissue involved by the tumor, has led to the emergence of a number of novel ablative methods, which are often specifically for unresectable primary or secondary carcinoma by inducing in situ coagulative necrosis (1,2). Cryoablation (cryosurgery) is one of the ablation methods.
The advent of newer delivery systems and imaging guidance, cryosurgery is performed during operation or via endoscopy (laparoscopy, bronchoscopy) or percutaneous approach, and has been used for treatment of malignant and benign tumors at a variety of sites. Specially, the minimally invasive cryoablation techniques led to wide application in oncology field (3).
Indication
Cryosurgery is adaptable for treatment of solid tumor of a variety of sites (Figure 1). The following diseases have been reported to be given cryosurgery (3,4).
Respiratory system
- ❖ Nonsmall cell lung cancer, small cell lung cancer;
- ❖ Carcinoid tumor of lungs, mesothelioma of pleura;
- ❖ Metastatic cancer of lungs.
Cardiovascular system
- ❖ Cardiac sarcoma;
- ❖ Metastatic cancer;
- ❖ Pericardial sarcoma.
Digestive system
- ❖ Hepatocellular carcinoma;
- ❖ Colorectal liver metastases;
- ❖ Noncolorectal liver metastases;
- ❖ Intrahepatic (peripheral) cholangiocarcinoma;
- ❖ Angiosarcoma of liver;
- ❖ Neuroendocrine tumor of liver;
- ❖ Focal nodular hyperplasia of liver;
- ❖ Pancreatic cancer;
- ❖ Anorectal carcinoma.
Urologic and male genital system
- ❖ Renal cell carcinoma;
- ❖ Bladder cancer;
- ❖ Prostate cancer;
- ❖ Testicular cancer;
- ❖ Tumor of adrenal gland.
Gynecologic system
- ❖ Cervical cancer;
- ❖ Uterine myomas;
- ❖ Uterine cancer;
- ❖ Ovarian cancer;
- ❖ Cervical intraepithelial neoplasia.
Head-neck region
- ❖ Oral cancer;
- ❖ Tongue cancer;
- ❖ Thyroid cancer;
- ❖ Nasopharyngeal cancer;
- ❖ Throat cancer;
- ❖ Leukoplakia, erythroplasia, lichen ruber planus.
Exterior of body
- ❖ Breast cancer;
- ❖ Breast fibroadenomas;
- ❖ Melanoma;
- ❖ Skin cancer (squamous cell carcinoma and basal cell carcinoma);
- ❖ Bowen disease;
- ❖ Vulvar cancer;
- ❖ Carcinoma of the penis.
Skeleton and soft tissue
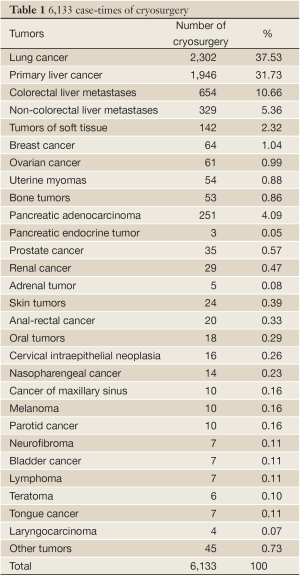
In the hospital where authors work during the period of from 2001 to 2010, there were more than 5,000 patients with cancer underwent a total of more than 6,000 case-times of cryosurgery (Table 1) (5).
Full table
- ❖ Simple bone cyst;
- ❖ Aneurysmal bone cyst;
- ❖ Giant cell tumor;
- ❖ Eosinophilic granuloma;
- ❖ Enchondroma and chondrosarcoma;
- ❖ Fibrous dysplasia, Fibroma and fibromatosis;
- ❖ Lipoma and liposarcoma;
- ❖ Angioma and lymphoangioma;
- ❖ Rhabdomyosarcoma;
- ❖ Leiomyosarcoma;
- ❖ Sarcoma of synovial membrane;
- ❖ Malignant nerve sheath tumor and Ewing’s sarcoma.
Usefulness
The techniques of present-day cryosurgery performed with multiprobe freezing apparatus and advanced imaging techniques yield predictable and encouraging results in the treatment of a variety of benign and malignant tumors, The procedure by which cryosurgery is performed includes intraoperative cryosurgery (open cryosurgery), endoscopic (laparoscopic, gastrointestinal endoscopic, bronchial endoscopic) cryosurgery, and percutaneous cryosurgery under imaging guidance (ultrasound, CT or MR imaging).
As a radical option
Cryosurgery may be used as an alternative of surgery, mainly is adaptable for (I) patients with unresectable cancer due to anatomic location; (II) patients who can not tolerate operation due to co-morbidity (poor general performance, cardio-pulmonary insufficiency); (III) patients who refuse operation due to psychological factor or cosmetic consideration (2).
Cryoablation for liver tumors
Patients with primary or metastatic liver tumors can be treated with percutaneous cryoablation (6). Cryoablation is highly effective in the treatment of liver tumors, the 5-year survival following rate cryoablation of tumors (<5 cm) which located far from large vessels is about 60%, which is similar to that following surgical resection. It is said that local recurrence rates after cryoablation is the lowest among all ablative therapies. Ultrasound-assisted intraoperative cryoablation has been reported to have local recurrence rates down to approximately 12%, compared with a 10% local recurrence rate for surgery alone (7). Recurrence rates are much higher for RF ablation. In one series of 117 patients with colorectal hepatic metastases treated by RF ablation, a 39% recurrence rate was reported (8).
Cryoablation for renal cancer
Cryoablation is the most evaluative probe ablative method for the treatment of small renal masses, is most commonly performed percutaneously or laparoscopically. Peripheral, posteriorly situated tumors arising from the inferior pole of the kidney are ideal candidates for this technique. Central tumors also can be treated with freezing without injury to the collecting system. With careful patient selection, the intermediate-term and long-term oncologic outcomes after cryoablation for kidney tumors showing 5- and 10-year cancer-specific survival are 93% and 81%, respectively (9,10).
Cryoablation for lung tumors
The majority of non-small cell lung cancer (NSCLC) patients with stage I/II disease are not surgical candidates due to co-morbid cardiopulmonary disease with insufficient reserve to withstand lobectomy. Systemic chemotherapy and/or external beam radiation have limited effect with a high local recurrence rate and poor long-term survival. Image-guided ablation techniques may be an alternative for patients with stage I/II disease not amenable to surgery. Percutaneous cryoablation has been proved to be a feasible option for unresectable small lung cancer (11). Cryoprobes can be placed close to the mediastinal and hilar vessels to intensify freezing because the collagenous architecture of the vessel wall is preserved, therefore, cryoablation may be preferable to radiofrequency (RF) ablation for centrally located lung cancer (3).
Cryoablation for the adrenal tumors
Adrenal tumors comprise a broad spectrum of benign and malignant neoplasms and include functional adrenal adenomas, pheochromocytomas, primary adrenocortical carcinoma, and adrenal metastases. Open and laparoscopic cryoablative approaches have been used in the treatment of adrenal tumors (12).
Cryoablation for prostate cancer
Prostate cryotherapy for localized prostate cancer is evolving. Using the American Society for Therapeutic Radiation and Oncology and the Phoenix (nadir plus 2) criteria for biochemical recurrence, Intermediate-term data show that primary cryotherapy appears to be comparable for low-risk prostate cancer as other treatment modalities (13). In addition, health-related quality-of-life measures have improved with the most recent third-generation systems demonstrating low rates of incontinence and urethrorectal fistula. Erectile dysfunction is high with whole gland ablation, but focal therapy may reduce these rates while still ablating unilateral cancerous tissue (14).
Cryoablation has emerged as an alternative minimally invasive salvage procedure after failure of primary radiation therapy. The negative biopsy for prostate cancer after cryotherapy is 80-90% at a median follow-up of 18 months. The factors that would predict an unfavorable outcome with salvage cryoablation, include a PSA level higher than 10 ng/mL before cryoablation, a Gleason score of 8 or more before radiation, stage T3/T4 disease, and probably those patients with increasing PSA levels despite hormonal therapy (15).
Cryoablation for breast cancer
Minimally invasive breast surgery is a broad concept encompassing new developments in the field of breast surgery that work on this minimally invasive principle. In this regard, breast-conserving surgery and sentinel lymph node biopsy are good illustrations of this concept. Percutaneous excisional devices are now available that can replace the surgical excision of breast mass lesions. Cryoablative treatments are capable of destroying breast cancers in situ instead of surgical excision. CT/US-guided multiprobe breast cryoablation safely achieved 1 cm visible ice beyond tumor margins with minimal discomfort, good cosmesis, and no short-term local tumor recurrences (16,17).
Cryoablation for fibroadenoma of the breast
Recent studies have demonstrated that, as a primary therapy for breast fibroadenoma, cryoablation is safe and effective with durable results and good cosmesis, that can be reproduced in community practices. US produced excellent ice visualization beyond tumor margins. Either removal or biopsy of a residual mass revealed a shrunken hyaline matrix with preserved collagenous architecture. Mammograms showed comparable resolution of mass effects with mild surrounding parenchymal reaction (18).
Cryoablation for bone tumors
The cryoablation suitable for bone tumor are divided into open and closed models. The following orthopedic bone diseases are suitable for cryosurgery: simple bone cyst, aneurysmal bone cyst, giant cell tumor, eosinophilic granuloma, enchondroma and chondrosarcoma grade 1, and fibrous dysplasia with commonly cured result (19). For malignant lesions of bone, cryosurgery can be used for marginal resection of the tumor which can not be resected due to its location or induces unacceptable morbidity, such as in vertebral (chordoma) and pelvic lesions (20,21).
As a complementary to surgery
The combination of cryoablation with a resective procedure expands the use of potentially curative procedures to bilobar liver cancer that is not readily amenable to standard surgical resection. For example, a patient with multiple lesions in the right lobe and a solitary lesion in the left lobe would not be a good candidate for either cryoablation alone or a standard resection, but the combination of a right hepatic lobectomy and cryoablation of the lesion in the left lobe could potentially result in better outcome. The patients with liver cancer associated with liver cirrhosis and decreased functional reserve of the liver can not survive an extensive hepatic resection, but may tolerate a lesser resection plus cryoablation, thus maximizing the remaining viable liver tissue and decreasing the overall morbidity and mortality of the procedure (22,23).
At the time of thoracotomy, the lung tumor was found to be more advanced than expected, or the patient who has poor respiratory function not fit for pneumonectomy or bilobectomy, the use of direct cryosurgery presents another option (24). The one or multiple cryoprobes are used for freezing all macroscopically visible tumors and a 5 mm margin of normal lung tissue.
For the large tumors, such as soft tissue tumor (fibroma and fibromatosis, liposarcoma, rhabdomyosarcoma and leiomyosarcoma), especially ones with rich vascularity, freezing the targeted tissue which is planned to be removed by operation, may decrease intraoperative bleeding (3).
It is suggested that combining cryosurgery with excision can be advantageous since freezing the tumor before excision minimizes the risk of spreading the cancerous cells during excision (25).
As one of comprehensive treatment for advanced cancer
Most local solid tumors can be treated with percutaneous cryoablation. These tumors include limited retroperitoneal lymphadenopathy from completely treated primaries such as renal cell carcinoma, focal intraperitoneal soft tissue metastases of ovarian carcinoma with no disease elsewhere, and recurrent rectal adenocarcinoma limited to the presacral region. For the metastatic cancer, percutaneous cryoablation may be used aiming to debulk tumor or largest tumor mass, and easier for chemotherapy or radiotherapy. It is important that cryosurgical procedures can be repeated as necessary in order to destroy all cancerous tissue (3).
Pancreatic cancer is a rapidly growing tumor that is nearly always fatal. The majority of pancreatic cancers are detected at a late stage of illness, and only a minority of patients is candidates for curative surgical resection. Overall, the 1- and 5-year survival rates are only 20% and 5%, respectively (26-29). Early 1980s, there were reports of using cryosurgery for locally advanced pancreatic cancer. Korpan (4) summarized the experience of cryosurgery for pancreatic cancer, and concluded that most patients obtained good results with this therapeutic modality. According to our data, patients with locally advanced pancreatic cancer underwent combination treatment in which percutaneous cryoablation was the main option had median survival of 16.2 mo, with 26 patients (53.1%) surviving for 12 mo or more. Overall, the 6-, 12-, 24- and 36-mo survival rates were 94.9%, 63.1%, 22.8% and 9.5%, respectively (30).
As a palliation modality
Cryoablation causes mild or insignificant pain compared with other ablation techniques, and provides long-lasting pain relief, hence it can be used effectively for pain palliation, and specially is adaptable for patients with metastatic soft tissue and bone tumors adjacent to important critical structures such as the spinal cord, sciatic nerve, and gastrointestinal and urinary organs (31,32).
Advantages relative to other ablation modalities
Interstitial tumor ablation aims to create a toxic local environment that completely ablates the targeted lesion with an appropriate margin of healthy tissue and to avoid damage to the remaining surrounding healthy tissue, as a subset of interventional oncology, has got rapid development. Now, 4 specific technologies [percutaneous ethanol injection (PEI), radiofrequency (RF) ablation, microwave ablation and cryoablation], are focused on.
Percutaneous ethanol ablation is the first ablative strategy to be widely deployed for liver tumors (33). However, PEI is generally only effective for hepatocellular carcinoma (HCC), not for other liver metastases. The reason is that HCC often is associated with liver cirrhosis, the hard, cirrhotic liver and the commonly present tumor capsule hold the ethanol within the soft liver tumor. Conversely, in most liver metastases the tumor is generally hard and surrounded by soft liver; in this setting, the injected ethanol usually takes the path of least resistance, following a fissure out of the lesion into the healthy surrounding liver, rather than being contained within and diffusing throughout the lesion. The primary disadvantages to PEI for HCC are that complete tumor necrosis can be obtained in about 75% of cases only, and decreases with increasing, and that many sessions required per patient with larger tumor (2). Now PEI for HCC is gradually being supplanted by RF ablation (34).
RF ablation utilizes high frequency, alternating current with a wavelength of 460-500 kHz. Flowing electrons induce frictional heating in cells near the site of energy emission. When living human tissues are heated above 49 °C cell death occurs within minutes (2). Temperatures in excess of 60 °C cause instantaneous cell death. RF ablation is relatively safe and simple to perform. An ideal tumor is one that is less than 3 cm in size, completely surrounded by liver parenchyma, away from the capsule and adjacent bowel, away from the large blood vessels, the gallbladder, and the central large bile duct. The disadvantages include the difficulty of monitoring the extent of the ablated zone during therapy by ultrasound or CT, a high local recurrence rate when applied percutaneously, and the heat and pain caused by RF ablation, which require general anesthesia in many cases (2,35,36).
Microwave ablation is a technique which inducing tumor destruction with higher frequency electromagnetic energy in the range of 900-2,450 MHz. Within this electromagnetic field, polar molecules (mainly water), try to align themselves in the direction of the current and as the direction changes constantly this continuous realignment causes a heating effect. Electromagnetic energy heats tissue by targeting the water molecules and induces cell death by coagulative necrosis (37,38). This ablation modality theoretically has many advantages over RF ablation, but is a relatively unproven technology with sparse clinical data for modern microwave devices.
Compared with heat-based modalities, including RF and microwave ablation, cryoablation has the following advantages:
One theoretical disadvantage of cryoablation relates to the risks of tumor seeding. Heat-based modalities, such as radiofrequency and microwave ablation, over cryoablation is the ability to cauterize the applicator track, so decrease tumor seeding, that is not possible with cryoablation (2). However, neither has been experimentally proven nor demonstrated in a clinical trial (49).
Another potential disadvantage to cryoablation is bleeding and rupture of ice ball. Bleeding has been considered an unusual complication in RF ablation, due to heat-based ablation modalities cause profound vascular thrombosis. In contrast, cryoablation has no intrinsic hemostatic properties and has rarely been associated with substantial hemorrhage during large-volume freezes performed at open laparotomy. But with use of new smaller probes sizes (1.7 mm) for cryoablation, this is not a clinically significant problem, except when freezing results in cracking of the liver capsule during thawing (50). Moreover, percutaneous cryoablation does not appear to a high bleeding rate, perhaps because percutaneous ablation does not have the iceball-air interface, is not performed in a low pressure environment, and has the benefit of surrounding tissues for tamponade (51-53).
Particularly the complication has been paid attention in HCC, because most of these patients have some level of bleeding diathesis and there is inherent cauterization associated with the heat-based therapies, but not with cryotherapy, that is why RF is advocated by some centers. But, the complications are mainly seen when use of cryoprobes as large as 10 mm. With improvements in technology, percutaneous cryoprobes as small as 17 gauge are now available for clinical use, the incidence of these complications has been decreased (3).
It is said that during the cryoablation, the systemic inflammatory marker concentrations (such as tumor necrosis factor-α and interleukin-1β) were significantly lower than those in the RF. The combination of rapid cell death with access to the systemic circulation may account for the cryoshock and multisystem failure (54,55). But these fateful complications were reported in the past when larger cryoablations (in treated volume, sometimes exceeding 35% of the liver) were performed with large size diameter of cryoprobe (54).
Conclusions
Cryoablation is adaptable for the treatment of nearly all solid tumors. According to current experience, percutaneous cryoablation, as a feasible and mini-invasive technique offers an effective and safe therapy for patients with tumors, and is especially suitable for the treatment of unresectable advanced tumors. The role of this technique is growing, with large-scale prospective randomized trials, continued advances can be expected.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Goldberg SN, Charboneau JW, Dodd GD 3rd, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology 2003;228:335-45.
- Kutikov A, Kunkle DA, Uzzo RG. Focal therapy for kidney cancer: a systematic review. Curr Opin Urol 2009;19:148-53.
- Xu KC, Niu LZ. eds. Cryosurgery for Cancer. Shanghai: Shanghai Sci-Tech-Edu Pub., 2007.
- Korpan NN. Basics of Cryosurgery. In: Korpan NN. eds. Pancreas cryosurgery. Wein NewYork: Springer-Verlag, 2001:151-4.
- Xu KC, Niu LZ, Hu YZ, et al. Clinical experience of Cryosurgery on 3,580 patients with solid tumors, Technol Cancer Res Treat 2007;6:450-1.
- Xu KC, Niu LZ, He WB, et al. Percutaneous cryoablation in combination with ethanol injection for unresectable hepatocellular carcinoma. World J Gastroenterol 2003;9:2686-9.
- Cha C, Lee FT Jr, Rikkers LF, et al. Rationale for the combination of cryoablation with surgical resection of hepatic tumors. J Gastrointest Surg 2001;5:206-13.
- Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159-66.
- Atwell TD, Callstrom MR, Farrell MA, et al. Percutaneous renal cryoablation: local control at mean 26 months of followup. J Urol 2010;184:1291-5.
- Weisbrod AJ, Atwell TD, Frank I, et al. Percutaneous cryoablation of masses in a solitary kidney. AJR Am J Roentgenol 2010;194:1620-5.
- Kawamura M, Izumi Y, Tsukada N, et al. Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates. J Thorac Cardiovasc Surg 2006;131:1007-13.
- Munver R, Sosa RE. Cryosurgery of the adrenal gland. Technol Cancer Res Treat 2004;3:181-5.
- Mouraviev V, Johansen TE, Polascik TJ. Contemporary results of focal therapy for prostate cancer using cryoablation. J Endourol 2010;24:827-34.
- Tsivian M, Polascik TJ. Focal cryotherapy for prostate cancer. Curr Urol Rep. 2010;11:147-51.
- Cox JM, Busby JE. Salvage therapy for prostate cancer recurrence after radiation therapy. Curr Urol Rep 2009;10:199-205.
- Littrup PJ, Jallad B, Chandiwala-Mody P, et al. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol 2009;20:1329-41.
- Tozaki M, Fukuma E, Suzuki T, et al. Ultrasound-guided cryoablation of invasive ductal carcinoma inside the MR room. Magn Reson Med Sci 2010;9:31-6.
- Kaufman CS, Littrup PJ, Freeman-Gibb LA, et al. Office-based cryoablation of breast fibroadenomas with long-term follow-up. Breast J 2005;11:344-50.
- Beeson J. Palliation of tracheobronchial carcinoma: the role of cryosurgery. J Perioper Pract 2007;17:332, 334-6, 338-9.
- Ullrick SR, Hebert JJ, Davis KW. Cryoablation in the musculoskeletal system. Curr Probl Diagn Radiol 2008;37:39-48.
- Mohler DG, Chiu R, McCall DA, et al. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin Orthop Relat Res 2010;468:2765-73.
- Xu KC, Niu LZ, He WB, et al. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol 2008;14:1430-6.
- Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: percutaneous and open approaches. J Surg Oncol 2009;100:619-34.
- Maiwand M. Endobronchial cryosurgery. Chest surg Clin N Am 2001;11:791-811.
- Freezing out cancer. Cryoablation could be a potential new treatment for a wider variety of cancers. Duke Med Health News 2010;16:3.
- Bast RC, Kufe DY, Pollock RE, et al. Cancer Medicine, 5th eds. In: Wolff RA, Abbruzzese JL, Evans DB, et al. eds. Neoplasms of the Exocrine Pancreas,” Singapore: Harcourt Asia Pte. Ltd., 2000:1436-64.
- Xu KC, Jiang SH. Modern Therapy of Digestive Disease. In: Xu KC, Xu P. eds. The treatment of pancreatic cancer. Shanghai: Shanghai Sci-Tech-Edu. Pub., 2001:618-24.
- Claude L, Mornex F. Chemoradiation in pancreatic carcinoma. Cancer Radiother 2003;7:254-65.
- Ducreux M, Boige V,Malka D. Treatment of advanced pancreatic cancer. Semin Oncol 2007;34:S25-S30.
- Xu KC, Niu LZ, Hu YZ, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol 2008;14:1603-11.
- Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol 2007;10:120-31.
- Lessard AM, Gilchrist J, Schaefer L, et al. Palliation of recurrent Ewing sarcoma of the pelvis with cryoablation and somatosensory-evoked potentials. J Pediatr Hematol Oncol 2009;31:18-21.
- Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology 1995;197:101-8.
- Rust C, Gores GJ. Locoregional management of hepatocellular carcinoma. Surgical and ablation therapies. Clin Liver Dis 2001;5:161-73.
- Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology 2010;78:113-24.
- McWilliams JP, Yamamoto S, Raman SS, et al. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol 2010;21:S204-13.
- Abbas G, Pennathur A, Landreneau RJ, et al. Radiofrequency and microwave ablation of lung tumors. J Surg Oncol 2009;100:645-50.
- Ryan TP, Turner PF, Hamilton B. Interstitial microwave transition from hyperthermia to ablation: historical perspectives and current trends in thermal therapy. Int J Hyperthermia 2010;26:415-33.
- Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol 2004;6:S9-S19.
- Leyendecker JR, Dodd GD 3rd, Halff GA, et al. Sonographically observed echogenic response during intraoperative radiofrequency ablation of cirrhotic livers: pathologic correlation. AJR Am J Roentgenol 2002;178:1147-51.
- Raman JD, Hall DW, Cadeddu JA. Renal ablative therapy: radiofrequency ablation and cryoablation. J Surg Oncol 2009;100:639-44.
- Winter TC, Laeseke PF, Lee FT Jr. Focal tumor ablation: a new era in cancer therapy. Ultrasound Q 2006;22:195-217.
- Tatli S, Acar M, Tuncali K, et al. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol 2010;16:90-5.
- Ritch CR, Katz AE. Prostate cryotherapy: current status. Curr Opin Urol 2009;19:177-81.
- Baust JG, Gage AA, Robilottto AT, et al. The pathophysiology of thermoablation: optimizing cryoablation. Curr Opin Urol 2009;19:127-32.
- Johnson JP. Immunologic aspects of cryosurgery: potential modulation of immune recognition and effector cell maturation. Clin Dermatol 1990;8:39-47.
- Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology 2002;60:40-9.
- Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009;58:1-11.
- Kramer BA, Whelan CM, Vestal JC, et al. Increasing the number of biopsy cores before renal cryoablation increases the diagnostic yield. J Endourol 2009;23:283-6.
- Weber SM, Lee FT Jr. Expanded treatment of hepatic tumors with radiofrequency ablation and cryoablation. Oncology (Williston Park) 2005;19:27-32.
- Adam R, Akpinar E, Johann M, et al. Place of cryosurgery in the treatment of malignant liver tumors. Ann Surg 1997;225:39-8; discussion 48-50.
- Lee FT Jr, Chosy SG, Littrup PJ, et al. CT-monitored percutaneous cryoablation in a pig liver model: pilot study. Radiology 1999;211:687-92.
- Harada J, Dohi M, Mogami T, et al. Initial experience of percutaneous renal cryosurgery under the guidance of a horizontal open MRI system. Radiat Med 2001;19:291-6.
- Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg 1999;23:109-13; discussion 113-4.
- Seifert JK, Achenbach T, Heintz A, et al. Cryotherapy for liver metastases. Int J Colorectal Dis 2000;15:161-6.